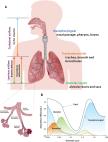
The COVID-19 pandemic has highlighted controversies and unknowns about how respiratory pathogens spread between hosts. Traditionally, it was thought that respiratory pathogens spread between people through large droplets produced in coughs and through contact with contaminated surfaces (fomites). However, several respiratory pathogens are known to spread through small respiratory aerosols, which can float and travel in air flows, infecting people who inhale them at short and long distances from the infected person. Wang et al . review recent advances in understanding airborne transmission gained from studying the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and other respiratory pathogens. The authors suggest that airborne transmission may be the dominant form of transmission for several respiratory pathogens, including SARS-CoV-2, and that further understanding of the mechanisms underlying infection from the airborne route will better inform mitigation measures. —GKA A Review discusses the scientific basis of and factors controlling airborne transmission of respiratory viruses including coronavirus. BACKGROUND Exposure to droplets produced in the coughs and sneezes of infected individuals or contact with droplet-contaminated surfaces (fomites) have been widely perceived as the dominant transmission modes for respiratory pathogens. Airborne transmission is traditionally defined as involving the inhalation of infectious aerosols or “droplet nuclei” smaller than 5 μm and mainly at a distance of >1 to 2 m away from the infected individual, and such transmission has been thought to be relevant only for “unusual” diseases. However, there is robust evidence supporting the airborne transmission of many respiratory viruses, including severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome (MERS)–CoV, influenza virus, human rhinovirus, and respiratory syncytial virus (RSV). The limitations of traditional views of droplet, fomite, and airborne transmission were illuminated during the COVID-19 pandemic. Droplet and fomite transmission of SARS-CoV-2 alone cannot account for the numerous superspreading events and differences in transmission between indoor and outdoor environments observed during the COVID-19 pandemic. Controversy surrounding how COVID-19 is transmitted and what interventions are needed to control the pandemic has revealed a critical need to better understand the airborne transmission pathway of respiratory viruses, which will allow for better-informed strategies to mitigate the transmission of respiratory infections. ADVANCES Respiratory droplets and aerosols can be generated by various expiratory activities. Advances in aerosol measurement techniques, such as aerodynamic and scanning mobility particle sizing, have shown that the majority of exhaled aerosols are smaller than 5 μm, and a large fraction are <1 μm for most respiratory activities, including those produced during breathing, talking, and coughing. Exhaled aerosols occur in multiple size modes that are associated with different generation sites and production mechanisms in the respiratory tract. Although 5 μm has been used historically to distinguish aerosols from droplets, the size distinction between aerosols and droplets should be 100 μm, which represents the largest particle size that can remain suspended in still air for more than 5 s from a height of 1.5 m, typically reach a distance of 1 to 2 m from the emitter (depending on the velocity of airflow carrying the aerosols), and can be inhaled. Aerosols produced by an infected individual may contain infectious viruses, and studies have shown that viruses are enriched in small aerosols (<5 μm). The transport of virus-laden aerosols is affected by the physicochemical properties of aerosols themselves and environmental factors, including temperature, relative humidity, ultraviolet radiation, airflow, and ventilation. Once inhaled, virus-laden aerosols can deposit in different parts of the respiratory tract. Larger aerosols tend to be deposited in the upper airway; however, smaller aerosols, although they can also be deposited there, can penetrate deep into the alveolar region of the lungs. The strong effect of ventilation on transmission, the distinct difference between indoor and outdoor transmission, well-documented long-range transmission, the observed transmission of SARS-CoV-2 despite the use of masks and eye protection, the high frequency of indoor superspreading events of SARS-CoV-2, animal experiments, and airflow simulations provide strong and unequivocal evidence for airborne transmission. Fomite transmission of SARS-CoV-2 has been found to be far less efficient, and droplets are only dominant when individuals are within 0.2 m of each other when talking. Although both aerosols and droplets can be produced by infected individuals during expiratory activities, droplets fall quickly to the ground or surfaces within seconds, leaving an enrichment of aerosols over droplets. The airborne pathway likely contributes to the spread of other respiratory viruses whose transmission was previously characterized as droplet driven. The World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) have officially acknowledged the inhalation of virus-laden aerosols as a main transmission mode in spreading COVID-19 at both short and long ranges in 2021. OUTLOOK Airborne transmission of pathogens has been vastly underappreciated, mostly because of an insufficient understanding about the airborne behavior of aerosols and at least partially because of the misattribution of anecdotal observations. Given the lack of evidence for droplet and fomite transmission and the increasingly strong evidence for aerosols in transmitting numerous respiratory viruses, we must acknowledge that airborne transmission is much more prevalent than previously recognized. Given all that we have learned about SARS-CoV-2 infection, the aerosol transmission pathway needs to be reevaluated for all respiratory infectious diseases. Additional precautionary measures must be implemented for mitigating aerosol transmission at both short and long ranges, with particular attention to ventilation, airflows, air filtration, UV disinfection, and mask fit. These interventions are critical tools for ending the current pandemic and preventing future outbreaks. Phases involved in airborne transmission of respiratory viruses. Virus-laden aerosols (<100 I1/4m) are first generated by an infected individual through expiratory activities, through which they are exhaled and transported in the environment. They may be inhaled by a potential host to initiate a new infection, provided that they remain infectious. In contrast to droplets (>100 I1/4m), aerosols can linger in air for hours and travel beyond 1 to 2 m from the infected individual who exhales them, causing new infections at both short and long ranges. CREDIT: N. CARY/ SCIENCE The COVID-19 pandemic has revealed critical knowledge gaps in our understanding of and a need to update the traditional view of transmission pathways for respiratory viruses. The long-standing definitions of droplet and airborne transmission do not account for the mechanisms by which virus-laden respiratory droplets and aerosols travel through the air and lead to infection. In this Review, we discuss current evidence regarding the transmission of respiratory viruses by aerosols—how they are generated, transported, and deposited, as well as the factors affecting the relative contributions of droplet-spray deposition versus aerosol inhalation as modes of transmission. Improved understanding of aerosol transmission brought about by studies of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection requires a reevaluation of the major transmission pathways for other respiratory viruses, which will allow better-informed controls to reduce airborne transmission.