- Record: found
- Abstract: found
- Article: found
Challenge in treating COVID‐19 associate pulmonary aspergillosis: Supratherapeutic voriconazole levels
letter
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Sir,
There is a cumulative evidence suggesting COVID‐19 victims are prone to COVID‐19 associated
pulmonary aspergillosis (CAPA). COVID‐19 itself and immunomodulatory medications,
such as corticosteroids and tocilizumab, compromise the immune system to an extent
that opportunistic infections complicate the course further.
1
In this letter, we aimed to highlight the relationship between inflammation and voriconazole
trough levels in COVID‐19 patients.
Voriconazole is recommended as the first‐line agent for the treatment of invasive
pulmonary aspergillosis (IPA).
1
Voriconazole is metabolized with cytochrome P450 (CYP450) isoenzymes (mainly with
CYP2C19 and lesser extent with CYP3A4) to voriconazole N‐oxide. Voriconazole reaches
to steady‐state trough concentrations approximately at the fifth day of administration.
Therapeutic drug monitoring for voriconazole is recommended because of the narrow
therapeutic index.
2
Voriconazole dose for IPA is recommended as 4 mg/kg every 12 h for maintenance, followed
by 6 mg/kg loading dose every 12 h in the first day. It was recommended that the trough
level of voriconazole should be between 1.5 and 5.5 mg/L. Voriconazole trough level
over 4.5–6 mg/L has been associated with hepatotoxicity.
3
The common side effects of voriconazole were defined as visual disturbances, fever,
nausea, rash, vomiting, chills, headache, abnormal liver function tests, and hallucinations.
4
Since the beginning of COVID‐19 pandemic, a total of 13 COVID‐19 patients were treated
with voriconazole for CAPA in our university hospital based on mycological, clinical,
and radiological findings. Among 13 patients, 12 (92.3%) were critically ill. All
patients, except one, had bacterial or viral coinfection in addition to CAPA. Plasma
voriconazole level measurements were performed with liquid chromatography‐triple quadrupole
mass spectrometer (Shimadzu LCMS‐8040). Two of those had a DDI with voriconazole (with
500‐mg intravenous clarithromycin twice daily and 80‐mg oral omeprazole daily), which
might contribute to high voriconazole trough levels due to their inhibitory effect
on CYP450 isoenzymes. However, the voriconazole level remained elevated despite discontinuation
of clarithromycin in one patient, suggesting a different mechanism. In five (41.7%)
critically ill patients, the trough level of voriconazole remained in the supratherapeutic
range despite a dose reduction of 100 mg/day. In summary, no associated factor was
detected for the explanation of higher voriconazole trough levels in 12 critically
ill patients. It was observed that COVID‐19 patients were more prone to high voriconazole
levels than non‐COVID‐19 patients. In four of 13 non‐COVID‐19 patients, the voriconazole
trough level was supratherapeutic. COVID‐19 and non‐COVID‐19 patients were comparable
in terms of gender (46.2% vs. 46.2%, p = 1.000), age [median (inter quartile range):
63 (51.5–69.5) vs. 66 (46.5–72.0) years, p = .960] and the number of comorbidities
[median (inter quartile range): 2 (1–3.5) vs. 3 (1–4), p = .724]. Fatality was 69.2%
versus 61.5% among COVID19 and non‐COVID19 patients, respectively (p = .680). Since
most of our patients were hospitalized in the intensive care unit, we were only able
to monitor increased transaminase levels, which is one of the common findings of voriconazole
toxicity. Transaminases levels were significantly higher in patients with COVID‐19
(53.8% vs. 7.7%, p = .030). There was no significant difference in the first CRP levels
between the groups [median (inter quartile range): 11.20 (6.84–15.19) vs. 7.78 (3.99–10.43),
p = .153]. Voriconazole levels were also significantly higher in the COVID‐19 group
[median (inter quartile range): 5.8 (4.75–6.75) vs. 2.4 (0.99–4.60), p = .001]. A
mild and positive correlation was determined between trough levels of voriconazole
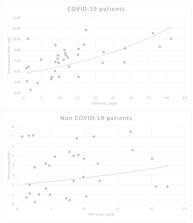
and C reactive protein (CRP) levels (r = 0.443, p < .001) (Figure 1).
FIGURE 1
The relationships of voriconazole and CRP levels in COVID‐19 and non COVID‐19 patients
For example, in a 57‐year‐old female patient (patient #11), despite the therapeutic
voriconazole level on day 6, an approximately twofold increase was measured on 20th
day of the treatment. Even though voriconazole dose was reduced to 100 mg per day,
its trough concentration was in 9.0 mg/L) on day 27. Therapeutic voriconazole trough
level range could be achieved 5 days after discontinuation of the treatment. A relation
between voriconazole trough levels and simultaneous (CRP) levels was observed in the
patient and no other explanatory reason for supratherapeutic levels of voriconazole
was found (Table 1).
TABLE 1
Disease and treatment characteristics of patients and voriconazole, and CRP levels
COVID‐19 patients
Patient #
Characteristics of patients
Weight (kg)
Voriconazole dosage
Route
Level and day of voriconazole
a
CRP (mg/dl)
The day of COVID‐19 when CAPA is diagnosed
1
67 years, male (critically ill)
80
320 mg q12h (2 × 480 mg loading)
iv
4.6 mg/L (6th day)
0.73
20th
8.2 mg/L (11th day)
15.30
2
77 years, male (critically ill)
60
200 mg q12h (2 × 300 mg loading)
iv
7.1 mg/L (5th day)
15.10
11th
5.6 mg/dl (6th day)
9.68
7.0 mg/dl (11th day)
11.75
3
78 years, male (critically ill)
70
280 mg q12h (2 × 420 mg loading)
iv
7.4 mg/dl (5th day)
11.55
38th
200 mg q12h
11.7 mg/dl (9th day)
17.40
2.2 mg/dl (5th day)
0.87
4
64 years, female (critically ill)
75
300 mg q12h (2 × 500 mg loading)
iv
5.8 mg/dl (6th day)
8.85
21st
5
70 years, female (critically ill)
80
320 mg q12h (2 × 480 mg loading)
iv
6.2 mg/dl (4th day)
4.83
15th
6
55 years, male (non‐critically ill)
65
200 mg q8h (2 × 400 mg loading)
po
4.9 mg/dl (5th day)
12.60
26th
0.6 mg/dl (48th day)
1.86
300 mg q12h
iv
3.0 mg/dl (55th day)
7.89
10.1 mg/dl (63rd day)
1.12
7
40 years, female (critically ill)
65
260 mg q12h (2 × 390 mg loading)
iv
5.6 mg/dl (8th day)
22.03
19th
210 mg q12h
iv
8.6 mg/dl (13th day)
38.00
Drug stopped
8
63 years, female (critically ill)
70
280 mg q12h (2 × 420 mg loading)
iv
6.4 mg/dl (4th day)
9.42
13th
250 mg q12h
iv
7.0 mg/dl (10th day)
9.55
9
61 years, female (critically ill)
75
360 mg q12h
iv
6.2 mg/dl (7th day)
11.20
9th
300 mg q12h
iv
8.3 mg/dl (14th day)
28.30
200 mg q8h
po
Drug stopped
1.8 mg/dl (20th day)
3.84
10
69 years, male (critically ill)
75
200 mg q8h (2 × 450 mg iv loading)
po
4.9 mg/dl (6th day)
1.27
23rd
5.7 mg/dl (12th day)
28.10
11
57 years, female (critically ill)
73
290 mg q12h (2 × 430 mg loading)
iv
3.0 mg/dl (6th day)
9.820
13th
6.6 mg/dl (20th day)
12.30
240 mg q12h
iv
9.0 mg/dl (27th day)
16.80
Drug stopped
10.1 mg/dl (28th day)
41.10
8.9 mg/dl (29th day)
8.87
1.9 mg/dl (33rd day)
20.20
12
48 years, male (critically ill)
70
280 mg q12h (2 × 420 mg loading)
iv
7.6 mg/dl (6th day)
22.30
18th
11.1 mg/dl (11th day)
36.10
Drug stopped
13
43 years, male (critically ill)
90
360 mg q12h (2 × 540 mg loading)
iv
3.0 mg/dl (3th day)
15.29
24th
2.6 mg/dl (12th day)
7.69
4.1 mg/dl (21th day)
8.68
8.0 mg/dl (28th day)
11.39
Drug stopped
Non‐COVID‐19 patients
Patient #
Characteristics of patients
Weight (kg)
Voriconazole dosage
Route
Level and day of voriconazole
a
CRP (mg/dl)
14
50 years, female (non‐critically ill)
75
200 mg q12h (2 × 450 mg iv loading)
po
0.97 mg/dl (7th day)
4.86
200 mg q8h
4.7 mg/dl (18th day)
20.10
15
66 years, male (critically ill)
50
200 mg q12h (2 × 400 mg iv loading)
po
1.8 mg/dl (3th day)
22.40
16
47 years, male (critically ill)
65
200 mg q12h (2 × 400 mg iv loading)
po
4.2 mg/dl (4th day)
12.00
17
46 years, male (critically ill)
80
320 mg q12h (2 × 480 mg loading)
iv
5.0 mg/dl (6th day)
8.40
18
56 years, female (critically ill)
50
200 mg q12h (2 × 400 mg iv loading)
po
2.4 mg/dl (15th day)
8.40
1.8 mg/dl (32th day)
20.70
1.6 mg/dl (50th day)
4.26
19
55 years, female (critically ill
60
240 mg q12h (2 × 360 mg loading)
iv
0.2 mg/dl (6th day)
2.61
0.8 mg/dl (8th day)
10.30
360 mg q12h
4.7 mg/dl (12th day)
9.94
20
75 years, male (critically ill)
50
200 mg q12h (2 × 400 mg iv loading)
po
1.0 mg/dl (13th day)
3.24
2.8 mg/dl (20th day)
9.84
0.68 mg/dl (27th day)
1.37
21
67 years, male (critically ill)
75
300 mg q12h (2 × 450 mg loading)
iv
5.4 mg/dl (2th day)
7.78
7.0 mg/dl (5th day)
0.73
240 mg q12h
4.2 mg/dl (18th day)
4.24
22
29 years, female (critically ill)
55
220 mg q12h (2 × 330 mg loading)
iv
2.4 mg/dl (3th day)
12.30
23
68 years, female (critically ill)
70
280 mg q12h (2 × 420 mg loading)
iv
0.6 mg/dl (13th day)
7.28
400 mg q12h
5.1 mg/dl (20th day)
9.11
24
46 years, male (critically ill)
70
280 mg q12h (2 × 420 mg loading)
iv
2.0 mg/dl (20th day)
1.82
1.1 mg/dl (27th day)
1.86
5.6 mg/dl (34th day)
17.20
0.4 mg/dl (41th day)
7.81
7.5 mg/dl (54th day)
16.90
Drug stopped
25
73 years, female (critically ill)
90
360 mg q12h (2 × 540 mg loading)
iv
6.8 mg/dl (5th day)
8.86
300 mg q12h
po
7.0 mg/dl (10th day)
11.40
200 mg q12h
4.9 mg/dl (16th day)
5.61
26
71 years, male (non‐critically ill)
87
350 mg q12h (2 × 525 mg loading)
iv
4.0 mg/dl (4th day)
4.75
po
7.1 mg/dl (14th day)
2.39
7.1 mg/dl (23th day)
1.74
300 mg q12h
3.8 mg/dl (34th day)
2.56
3.4 mg/dl (42th day)
1.05
Abbreviations: CRP: C reactive protein; iv: intravenous.
a
Trough levels of voriconazole was performed by liquid chromatography–tandem mass spectrometry
(LC‐MS/MS) assays with the EUREKA® kit.
Pharmacokinetics of voriconazole is affected by many factors such as impaired liver
functions, drug–drug interactions (DDIs), body‐weight of the patient, genetic polymorphism,
and food intake in oral use. Regardless, inflammatory cytokines may inhibit the activity
of enzymes by causing downregulation of CYP450 isoenzymes. Van Wanrooy et al. previously
reported higher voriconazole trough levels in patients with higher CRP values. Voriconazole
trough levels were elevated 0.015 mg/L for each 1 mg/L increase in CRP level.
5
Although the possible mechanism of inflammatory cytokines on cytochrome P450 isoenzymes
is not clear, microRNA (miRNA) molecules may play a role in the regulation of gene
expression. In general, binding of miRNA molecules and small noncoding RNAs to recognition
sites on target mRNA, leads to inhibition of the translation and transcription pathways
of gene expression. It was observed that levels of miRNA‐21 and miRNA‐130b were increased
during inflammation.
6
We suggest that in severe COVID‐19 patients with a high‐level inflammation, the risk
of voriconazole toxicity may be higher. The fact that the trough level of voriconazole
does not decrease despite the reduction of voriconazole dose could be explained by
the decrease in hepatic clearance due to genetic polymorphism or inflammation. Since
the presence of genetic polymorphism is not very common, inflammation may be the possible
triggered factor in these patients. Therefore, voriconazole levels may be monitored
more frequently in COVID‐19 patients, especially during the inflammatory phase. Additional
pharmacokinetic studies are warranted in COVID‐19 patient population to determine
the appropriate dosing of voriconazole to assure therapeutic trough levels.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Related collections
Most cited references6
- Record: found
- Abstract: found
- Article: not found
Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance
- Record: found
- Abstract: found
- Article: not found
Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper #
- Record: found
- Abstract: found
- Article: not found
The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial.
Kee-Seok Nam, G. N. Kim, Wan Beom Park … (2012)
