- Record: found
- Abstract: found
- Article: found
Low dose novel PARP-PI3K inhibition via nanoformulation improves colorectal cancer immunoradiotherapy
Read this article at
Abstract
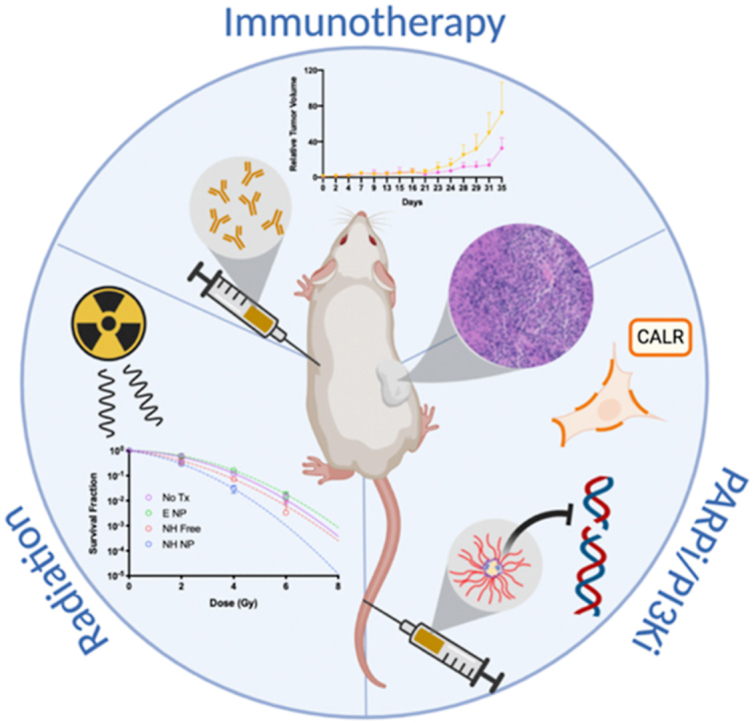
Multimodal therapy is often used in oncology to overcome dosing limitations and chemoresistance. Recently, combination immunoradiotherapy has shown great promise in a select subset of patients with colorectal cancer (CRC). Furthermore, molecularly targeted agents delivered in tandem with immunotherapy regimens have been suggested to improve treatment outcomes and expand the population of responding patients. In this study, radiation-sensitizing small molecules niraparib (PARP inhibitor) and HS-173 (PI3K inhibitor) are identified as a novel combination that synergistically enhance toxicity and induce immunogenic cell death both in vitro and in vivo in a CRC model. These inhibitors were co-encapsulated in a polymer micelle to overcome solubility limitations while minimizing off-target toxicity. Mice bearing syngeneic colorectal tumors (CT26) were administered these therapeutic micelles in combination with X-ray irradiation and anti-CTLA-4 immunotherapy. This combination led to enhanced efficacy demonstrated by improved tumor control and increased tumor infiltrating lymphocytes. This report represents the first investigation of DNA damage repair inhibition combined with radiation to potentiate anti-CTLA-4 immunotherapy in a CRC model.
Graphical abstract
Highlights
-
•
A novel combination of PARP and PI3K inhibitors induce radiosensitization in vitro and immunogenic cell death in vitro and in vivo.
-
•
Encapsulation of both niraparib and HS-173 in poly(2-oxazoline) micelles allows for co-delivery to colorectal cancer tumors
-
•
This nanoformulation combined with radiation improve CTLA-4 therapy by tumor growth delay and potentially increase the responding population.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Drug combination studies and their synergy quantification using the Chou-Talalay method.
- Record: found
- Abstract: found
- Article: not found
Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability–High Metastatic Colorectal Cancer
- Record: found
- Abstract: found
- Article: not found