- Record: found
- Abstract: found
- Article: found
Transthyretin Amyloidosis: Chaperone Concentration Changes and Increased Proteolysis in the Pathway to Disease
Read this article at
Abstract
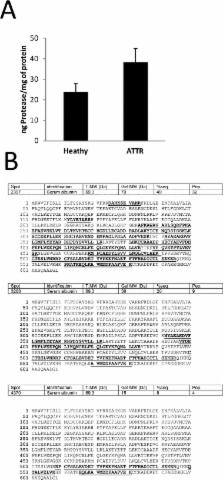
Transthyretin amyloidosis is a conformational pathology characterized by the extracellular formation of amyloid deposits and the progressive impairment of the peripheral nervous system. Point mutations in this tetrameric plasma protein decrease its stability and are linked to disease onset and progression. Since non-mutated transthyretin also forms amyloid in systemic senile amyloidosis and some mutation bearers are asymptomatic throughout their lives, non-genetic factors must also be involved in transthyretin amyloidosis. We discovered, using a differential proteomics approach, that extracellular chaperones such as fibrinogen, clusterin, haptoglobin, alpha-1-anti-trypsin and 2-macroglobulin are overrepresented in transthyretin amyloidosis. Our data shows that a complex network of extracellular chaperones are over represented in human plasma and we speculate that they act synergistically to cope with amyloid prone proteins. Proteostasis may thus be as important as point mutations in transthyretin amyloidosis.
Related collections
Most cited references54
- Record: found
- Abstract: not found
- Article: not found
A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves.
- Record: found
- Abstract: found
- Article: not found
Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia.
- Record: found
- Abstract: found
- Article: not found