- Record: found
- Abstract: found
- Article: found
HDAC3 Activity is Essential for Human Leukemic Cell Growth and the Expression of β-catenin, MYC, and WT1
Read this article at
Abstract
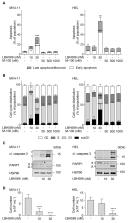
Therapy of acute myeloid leukemia (AML) is unsatisfactory. Histone deacetylase inhibitors (HDACi) are active against leukemic cells in vitro and in vivo. Clinical data suggest further testing of such epigenetic drugs and to identify mechanisms and markers for their efficacy. Primary and permanent AML cells were screened for viability, replication stress/DNA damage, and regrowth capacities after single exposures to the clinically used pan-HDACi panobinostat (LBH589), the class I HDACi entinostat/romidepsin (MS-275/FK228), the HDAC3 inhibitor RGFP966, the HDAC6 inhibitor marbostat-100, the non-steroidal anti-inflammatory drug (NSAID) indomethacin, and the replication stress inducer hydroxyurea (HU). Immunoblotting was used to test if HDACi modulate the leukemia-associated transcription factors β-catenin, Wilms tumor (WT1), and myelocytomatosis oncogene (MYC). RNAi was used to delineate how these factors interact. We show that LBH589, MS-275, FK228, RGFP966, and HU induce apoptosis, replication stress/DNA damage, and apoptotic fragmentation of β-catenin. Indomethacin destabilizes β-catenin and potentiates anti-proliferative effects of HDACi. HDACi attenuate WT1 and MYC caspase-dependently and -independently. Genetic experiments reveal a cross-regulation between MYC and WT1 and a regulation of β-catenin by WT1. In conclusion, reduced levels of β-catenin, MYC, and WT1 are molecular markers for the efficacy of HDACi. HDAC3 inhibition induces apoptosis and disrupts tumor-associated protein expression.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML.
- Record: found
- Abstract: found
- Article: not found
HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner.
- Record: found
- Abstract: found
- Article: not found