- Record: found
- Abstract: not found
- Article: not found
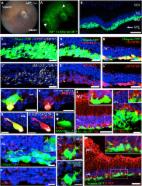
Assessment of AAV Vector Tropisms for Mouse and Human Pluripotent Stem Cell–Derived RPE and Photoreceptor Cells
Anai Gonzalez-Cordero
1 ,
Debbie Goh
1 ,
Kamil Kruczek
1 ,
Arifa Naeem
1 ,
Milan Fernando
1 ,
Sophia-Martha kleine Holthaus
1
,
2 ,
Matsuki Takaaki
1 ,
Samuel J. I. Blackford
1 ,
Magdalena Kloc
1 ,
Leticia Agundez
1 ,
Robert D. Sampson
1 ,
Shyamanga Borooah
3 ,
Patrick Ovando-Roche
1 ,
Manjit S. Mehat
1 ,
Emma L. West
1 ,
Alexander J. Smith
1 ,
Rachael A. Pearson
1 ,
Robin R. Ali
1
October 2018
October 2018
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Modeling early retinal development with human embryonic and induced pluripotent stem cells.
- Record: found
- Abstract: found
- Article: found
Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors
Anai Gonzalez‐Cordero, Kamil Kruczek, Arifa Naeem … (2017)
- Record: found
- Abstract: found
- Article: not found
iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration.
David Gamm, B Pattnaik, Wei Shen … (2013)
