- Record: found
- Abstract: found
- Article: found
Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7
Read this article at
Abstract
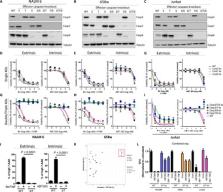
Caspase-3 and -7 are redundantly required to amplify the extrinsic and intrinsic apoptotic cascade in human leukemia.
Abstract
Apoptosis is a complex multi-step process driven by caspase-dependent proteolytic cleavage cascades. Dysregulation of apoptosis promotes tumorigenesis and limits the efficacy of chemotherapy. To assess the complex interactions among caspases during apoptosis, we disrupted caspase-8, -9, -3, -7, or -6 and combinations thereof, using CRISPR-based genome editing in living human leukemia cells. While loss of apical initiator caspase-8 or -9 partially blocked extrinsic or intrinsic apoptosis, respectively, only combined loss of caspase-3 and -7 fully inhibited both apoptotic pathways, with no discernible effect of caspase-6 deficiency alone or in combination. Caspase-3/7 double knockout cells exhibited almost complete inhibition of caspase-8 or -9 activation. Furthermore, deletion of caspase-3 and -7 decreased mitochondrial depolarization and cytochrome c release upon apoptosis activation. Thus, activation of effector caspase-3 or -7 sets off explosive feedback amplification of upstream apoptotic events, which is a key feature of apoptotic signaling essential for efficient apoptotic cell death.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis.
- Record: found
- Abstract: found
- Article: not found
Caspases 3 and 7: key mediators of mitochondrial events of apoptosis.
- Record: found
- Abstract: found
- Article: not found