- Record: found
- Abstract: found
- Article: found
Lymphatic and Immune Cell Cross-Talk Regulates Cardiac Recovery After Experimental Myocardial Infarction
Read this article at
Abstract
Supplemental Digital Content is available in the text.
Abstract
Objective:
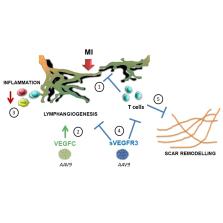
Lymphatics play an essential pathophysiological role in promoting fluid and immune cell tissue clearance. Conversely, immune cells may influence lymphatic function and remodeling. Recently, cardiac lymphangiogenesis has been proposed as a therapeutic target to prevent heart failure after myocardial infarction (MI). We investigated the effects of gene therapy to modulate cardiac lymphangiogenesis post-MI in rodents. Second, we determined the impact of cardiac-infiltrating T cells on lymphatic remodeling in the heart.
Approach and Results:
Comparing adenoviral versus adeno-associated viral gene delivery in mice, we found that only sustained VEGF (vascular endothelial growth factor)-C C156S therapy, achieved by adeno-associated viral vectors, increased cardiac lymphangiogenesis, and led to reduced cardiac inflammation and dysfunction by 3 weeks post-MI. Conversely, inhibition of VEGF-C/-D signaling, through adeno-associated viral delivery of soluble VEGFR3 (vascular endothelial growth factor receptor 3), limited infarct lymphangiogenesis. Unexpectedly, this treatment improved cardiac function post-MI in both mice and rats, linked to reduced infarct thinning due to acute suppression of T-cell infiltration. Finally, using pharmacological, genetic, and antibody-mediated prevention of cardiac T-cell recruitment in mice, we discovered that both CD4 + and CD8 + T cells potently suppress, in part through interferon-γ, cardiac lymphangiogenesis post-MI.
Conclusions:
We show that resolution of cardiac inflammation after MI may be accelerated by therapeutic lymphangiogenesis based on adeno-associated viral gene delivery of VEGF-C C156S. Conversely, our work uncovers a major negative role of cardiac-recruited T cells on lymphatic remodeling. Our results give new insight into the interconnection between immune cells and lymphatics in orchestration of cardiac repair after injury.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
The inflammatory response in myocardial injury, repair, and remodelling.
- Record: found
- Abstract: found
- Article: found
Development and plasticity of meningeal lymphatic vessels
- Record: found
- Abstract: found
- Article: not found