- Record: found
- Abstract: found
- Article: found
Atualização dos rastreadores para detecção de eventos adversos a medicamentos em pacientes hematológicos Translated title: Update of triggers for detection of adverse drug events in hematologic patients Translated title: Actualización de los rastreadores para detectar eventos farmacológicos adversos en pacientes hematológicos
Resumo:
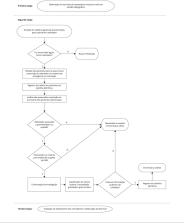
A utilização de rastreadores para a busca ativa e detecção de eventos adversos a medicamentos (EAM) tem ganhado espaço nos serviços de farmacovigilância. Assim, o objetivo principal do estudo foi propor uma nova lista de rastreadores para ser empregada em um centro especializado em hematologia do Rio de Janeiro, Brasil. A atualização da lista de rastreadores consistiu na revisão da lista atual, com a exclusão e inclusão de rastreadores. Para verificar o desempenho da nova lista de rastreadores, realizou-se um estudo transversal em que os novos rastreadores foram utilizados para investigar a ocorrência de EAM em pacientes atendidos na emergência ou hospitalizados no período de janeiro a março de 2022. Para cada suspeita de EAM identificada, caracterizaram-se o perfil do paciente e as reações adversas a medicamentos (RAM) quanto à causalidade e gravidade. O desempenho dos rastreadores e sua capacidade de captação de EAM foram calculados por meio dos indicadores: frequência do rastreador por 100 prontuários, frequência de EAM por 100 prontuários e valor preditivo positivo (VPP). Para avaliar o desempenho global da nova lista proposta, calculou-se o VPP. Foram identificadas 374 prescrições de rastreadores em 186 prontuários. Os mais eficientes na detecção de possíveis EAM foram: lidocaína, loperamida, bisacodil, filgrastim e clister de glicerina. O VPP global da nova lista sugerida foi 48% contra 10% da lista anterior. Este estudo demonstrou a importância de uma lista de rastreadores atualizada para o monitoramento dos EAM e o aprimoramento da assistência prestada.
Abstract:
The use of triggers for the active search and detection of adverse drug events (ADEs) has been gaining ground within pharmacovigilance services. Thus, the main objective of the study was to propose a new list of triggers to be used in a center specialized in hematology in Rio de Janeiro, Brazil. The update of the list of triggers consisted of revising the current list, with the exclusion and inclusion of new triggers. To verify the performance of the new list of triggers, a cross-sectional study was conducted in which the new triggers were used to investigate the occurrence of ADEs in patients attended in the emergency unit or hospitalized from January to March 2022. For each suspected ADEs, the patient’s profile and adverse drug reactions (ADRs) were characterized regarding causality and severity. The performance of the triggers and their ability to capture ADEs were estimated using the following indicators: frequency of the trigger per 100 medical records, frequency of ADEs per 100 records, and positive predictive value (PPV). To evaluate the overall performance of the proposed new list, the PPV was estimated. A total of 374 prescriptions for triggers were identified in 186 medical records. The most efficient in the detection of possible ADEs were: lidocaine, loperamide, bisacodyl, filgrastim and glycerin clyster. The overall PPV of the new suggested list was 48% versus 10% of the previous list. This study demonstrated the importance of an updated list of triggers for the monitoring of ADEs and improvement of the care provided.
Resumen:
El uso de rastreadores en la búsqueda y detección activa de eventos adversos de medicamentos (EAM) viene ganando espacio dentro de los servicios de farmacovigilancia. Por lo tanto, el objetivo principal de este estudio fue proponer una nueva lista de rastreadores que se utilizarán en un centro de hematología especializado en Río de Janeiro, Brasil. La actualización de la lista de rastreadores consistió en la revisión de la lista actual, con la exclusión e inclusión de rastreadores. Para verificar el desempeño de la nueva lista de rastreadores, se realizó un estudio transversal en el que se utilizaron los nuevos rastreadores para investigar la aparición de EAM en pacientes atendidos en urgencias u hospitalizados en el periodo de enero a marzo de 2022. Para cada sospecha de EAM identificados, el perfil del paciente y las reacciones adversas a medicamentos (RAM) se caracterizaron por su causalidad y gravedad. El desempeño de los rastreadores y su capacidad para capturar EAM se calcularon mediante los indicadores: frecuencia del rastreador por cada 100 registros médicos, frecuencia de EAM por cada 100 registros médicos y valor predictivo positivo (VPP). Para evaluar el desempeño general de la nueva lista propuesta, se calculó el VPP. Se identificaron 374 recetas de rastreadores en 186 registros médicos. Los más eficientes en la detección de posibles EAM fueron lidocaína, loperamida, bisacodilo, filgrastim y enema de glicerina. El VPP general de la nueva lista sugerida fue del 48% frente al 10% de la lista anterior. Este estudio demostró la importancia de una lista actualizada de rastreadores para monitorear EAM y mejorar la atención brindada.
Related collections
Most cited references38
- Record: found
- Abstract: not found
- Article: not found
A method for estimating the probability of adverse drug reactions.
- Record: found
- Abstract: not found
- Article: not found
A method for estimating the probability of adverse drug reactions
- Record: found
- Abstract: found
- Article: not found