- Record: found
- Abstract: found
- Article: found
Metal Ion Binding to the Amyloid β Monomer Studied by Native Top-Down FTICR Mass Spectrometry

Read this article at
Abstract
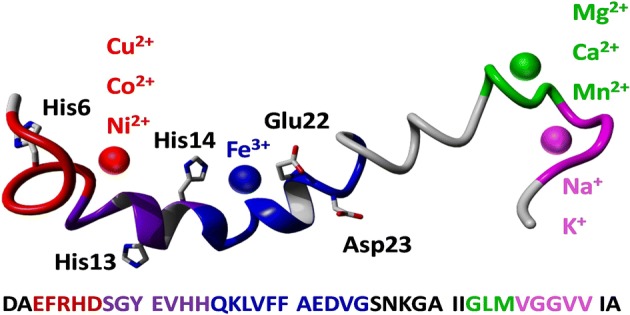
Native top-down mass spectrometry is a fast, robust biophysical technique that can provide molecular-scale information on the interaction between proteins or peptides and ligands, including metal cations. Here we have analyzed complexes of the full-length amyloid β (1-42) monomer with a range of (patho)physiologically relevant metal cations using native Fourier transform ion cyclotron resonance mass spectrometry and three different fragmentation methods—collision-induced dissociation, electron capture dissociation, and infrared multiphoton dissociation—all yielding consistent results. Amyloid β is of particular interest as its oligomerization and aggregation are major events in the etiology of Alzheimer’s disease, and it is known that interactions between the peptide and bioavailable metal cations have the potential to significantly damage neurons. Those metals which exhibited the strongest binding to the peptide (Cu 2+, Co 2+, Ni 2+) all shared a very similar binding region containing two of the histidine residues near the N-terminus (His6, His13). Notably, Fe 3+ bound to the peptide only when stabilized toward hydrolysis, aggregation, and precipitation by a chelating ligand, binding in the region between Ser8 and Gly25. We also identified two additional binding regions near the flexible, hydrophobic C-terminus, where other metals (Mg 2+, Ca 2+, Mn 2+, Na +, and K +) bound more weakly—one centered on Leu34, and one on Gly38. Unexpectedly, collisional activation of the complex formed between the peptide and [Co III(NH 3) 6] 3+ induced gas-phase reduction of the metal to Co II, allowing the peptide to fragment via radical-based dissociation pathways. This work demonstrates how native mass spectrometry can provide new insights into the interactions between amyloid β and metal cations.
Related collections
Most cited references88
- Record: found
- Abstract: found
- Article: not found
Studying noncovalent protein complexes by electrospray ionization mass spectrometry.
- Record: found
- Abstract: found
- Article: not found
Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence.
- Record: found
- Abstract: found
- Article: not found