- Record: found
- Abstract: found
- Article: found
Inflammasome Activation Induced by a Snake Venom Lys49-Phospholipase A 2 Homologue
Read this article at
Abstract
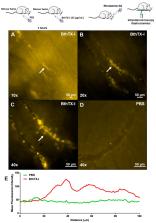
Background: Snake venom phospholipases A 2 (PLA 2s) have hemolytic, anticoagulant, myotoxic, oedematogenic, bactericidal, and inflammatory actions. BthTX-I, a Lys49-PLA 2 isolated from Bothrops jararacussu venom, is an example of Lys49-PLA 2 that presents such actions. NLRP3 is a cytosolic receptor from the NLR family responsible for inflammasome activation via caspase-1 activation and IL-1β liberation. The study of NLRs that recognize tissue damage and activate the inflammasome is relevant in envenomation. Methods: Male mice (18–20 g) received an intramuscular injection of BthTX-I or sterile saline. The serum was collected for creatine-kinase (CK), lactate dehydrogenase (LDH), and interleukin-1β (IL-1β) assays, and muscle was removed for inflammasome activation immunoblotting and qRT-PCR expression for nucleotide and oligomerization domain, leucine-rich repeat-containing protein family, pyrin-containing domain 3 receptor (NLRP3) inflammasome components. Results: BthTX-I-induced inflammation and myonecrosis, shown by intravital microscope, and LDH and CK release, respectively. Mouse treatment with A438079, a P2X7 receptor antagonist, did not modify these effects. BthTX-I induced inflammasome activation in muscle, but P2X7R participation in this effect was not observed. Conclusion: Together, the results showed for the first time that BthTX-I in gastrocnemius muscle induces inflammation and consequently, inflammasome activation via NLRP3 with caspase-1 activation and IL-1β liberation.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Nucleotide signalling during inflammation.
- Record: found
- Abstract: found
- Article: not found
ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way.
- Record: found
- Abstract: found
- Article: not found