- Record: found
- Abstract: found
- Article: found
Examining the use of the FIGO Nutrition Checklist in routine antenatal practice: multistakeholder feedback to implementation

Read this article at
Abstract
Objective
To gain insights from pregnant women and obstetricians on the utility of the FIGO Nutrition Checklist in antenatal practice.
Methods
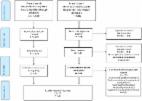
Women were recruited from the antenatal department of a large tertiary‐level university maternity hospital in Dublin, Ireland, between October and December 2019. Participants completed the FIGO Nutrition Checklist before their routine antenatal appointment. Obstetricians and women were encouraged to discuss the FIGO Nutrition Checklist during the clinical visit. Completed FIGO Nutrition Checklists were collected after appointments. Acceptability was assessed through questionnaires.
Results
The majority (80.0%) of women answered “No” to at least one diet quality question, indicating a potential nutritional risk. While none of the participating obstetricians routinely discussed nutrition with women, all agreed that using the Checklist encouraged them to address nutrition with pregnant women. Nearly every woman (99.0%) found the Checklist quick to complete; however, all participating obstetricians felt there was not enough time to discuss it in routine practice. Despite this, most obstetricians and pregnant women recommended the FIGO Nutrition Checklist for use.
Abstract
The FIGO Nutrition Checklist is acceptable to pregnant women and may capture high‐risk weight or dietary practices in pregnancy that would otherwise go untreated.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: found
Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework

- Record: found
- Abstract: found
- Article: found
CONSORT 2010 statement: extension to randomised pilot and feasibility trials
