- Record: found
- Abstract: found
- Article: found
The right time for senescence
Read this article at
Abstract
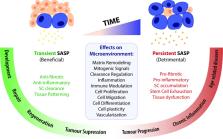
Cellular senescence is a highly complex and programmed cellular state with diverse and, at times, conflicting physiological and pathological roles across the lifespan of an organism. Initially considered a cell culture artifact, senescence evolved from an age-related circumstance to an intricate cellular defense mechanism in response to stress, implicated in a wide spectrum of biological processes like tissue remodelling, injury and cancer. The development of new tools to study senescence in vivo paved the way to uncover its functional roles in various frameworks, which are sometimes hard to reconcile. Here, we review the functional impact of senescent cells on different organismal contexts. We provide updated insights on the role of senescent cells in tissue repair and regeneration, in which they essentially modulate the levels of fibrosis and inflammation, discussing how “time” seems to be the key maestro of their effects. Finally, we overview the current clinical research landscape to target senescent cells and contemplate its repercussions on this fast-evolving field.
Related collections
Most cited references123
- Record: found
- Abstract: found
- Article: not found
Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.
- Record: found
- Abstract: found
- Article: not found
Induction of pluripotent stem cells from adult human fibroblasts by defined factors.
- Record: found
- Abstract: found
- Article: not found