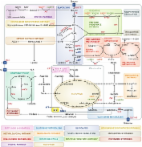
Nrf2:INrf2 (Keap1) are cellular sensors of oxidative and electrophilic stress. Nrf2 is a nuclear factor that controls the expression and coordinated induction of a battery of genes that encode detoxifying enzymes, drug transporters, antiapoptotic proteins, and proteasomes. In the basal state, Nrf2 is constantly degraded in the cytoplasm by its inhibitor, INrf2. INrf2 functions as an adapter for Cul3/Rbx1 E3 ubiquitin ligase-mediated degradation of Nrf2. Chemicals, including antioxidants, tocopherols including α-tocopherol (vitamin E), and phytochemicals, and radiation antagonize the Nrf2:INrf2 interaction and lead to the stabilization and activation of Nrf2. The signaling events involve preinduction, induction, and postinduction responses that tightly control Nrf2 activation and repression back to the basal state. Oxidative/electrophilic signals activate unknown tyrosine kinases in a preinduction response that phosphorylates specific residues on Nrf2 negative regulators, INrf2, Fyn, and Bach1, leading to their nuclear export, ubiquitination, and degradation. This prepares nuclei for unhindered import of Nrf2. Oxidative/electrophilic modification of INrf2 cysteine 151 followed by PKC phosphorylation of Nrf2 serine 40 in the induction response results in the escape or release of Nrf2 from INrf2. Nrf2 is thus stabilized and translocates to the nucleus, resulting in a coordinated activation of gene expression. This is followed by a postinduction response that controls the "switching off" of Nrf2-activated gene expression. GSK3β, under the control of AKT and PI3K, phosphorylates Fyn, leading to Fyn nuclear localization. Fyn phosphorylates Nrf2 Y568, resulting in nuclear export and degradation of Nrf2. The activation and repression of Nrf2 provide protection against oxidative/electrophilic stress and associated diseases, including cancer. However, deregulation of INrf2 and Nrf2 due to mutations may lead to nuclear accumulation of Nrf2 that reduces apoptosis and promotes oncogenesis and drug resistance. Copyright © 2013 Elsevier Inc. All rights reserved.