- Record: found
- Abstract: found
- Article: found
Development of a human in vitro blood–brain tumor barrier model of diffuse intrinsic pontine glioma to better understand the chemoresistance
Read this article at
Abstract
Background
Pediatric diffuse intrinsic pontine glioma (DIPG) represents one of the most devastating and lethal brain tumors in children with a median survival of 12 months. The high mortality rate can be explained by the ineligibility of patients to surgical resection due to the diffuse growth pattern and midline localization of the tumor. While the therapeutic strategies are unfortunately palliative, the blood–brain barrier (BBB) is suspected to be responsible for the treatment inefficiency. Located at the brain capillary endothelial cells (ECs), the BBB has specific properties to tightly control and restrict the access of molecules to the brain parenchyma including chemotherapeutic compounds. However, these BBB specific properties can be modified in a pathological environment, thus modulating brain exposure to therapeutic drugs. Hence, this study aimed at developing a syngeneic human blood–brain tumor barrier model to understand how the presence of DIPG impacts the structure and function of brain capillary ECs.
Methods
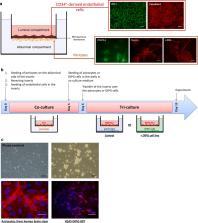
A human syngeneic in vitro BBB model consisting of a triple culture of human (ECs) (differentiated from CD34 +-stem cells), pericytes and astrocytes was developed. Once validated in terms of BBB phenotype, this model was adapted to develop a blood–brain tumor barrier (BBTB) model specific to pediatric DIPG by replacing the astrocytes by DIPG-007, -013 and -014 cells. The physical and metabolic properties of the BBTB ECs were analyzed and compared to the BBB ECs. The permeability of both models to chemotherapeutic compounds was evaluated.
Results
In line with clinical observation, the integrity of the BBTB ECs remained intact until 7 days of incubation. Both transcriptional expression and activity of efflux transporters were not strongly modified by the presence of DIPG. The permeability of ECs to the chemotherapeutic drugs temozolomide and panobinostat was not affected by the DIPG environment.
Conclusions
This original human BBTB model allows a better understanding of the influence of DIPG on the BBTB ECs phenotype. Our data reveal that the chemoresistance described for DIPG does not come from the development of a “super BBB”. These results, validated by the absence of modification of drug transport through the BBTB ECs, point out the importance of understanding the implication of the different protagonists in the pathology to have a chance to significantly improve treatment efficiency.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
The blood–brain barrier and blood–tumour barrier in brain tumours and metastases
- Record: found
- Abstract: found
- Article: not found
Blood-brain barrier structure and function and the challenges for CNS drug delivery.
- Record: found
- Abstract: found
- Article: not found