- Record: found
- Abstract: found
- Article: found
Carfilzomib-associated renal toxicity is common and unpredictable: a comprehensive analysis of 114 multiple myeloma patients
Read this article at
Abstract
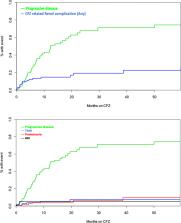
Carfilzomib (CFZ) is a non-reversible proteasome inhibitor approved for the treatment of patients with relapsed and refractory myeloma (RRMM). Its use has been associated with cardiovascular toxicity but although recently a signal of clinically significant renal complications has also been identified, it is less extensively investigated. We analyzed data of 114 consecutive patients with RRMM who received CFZ-based regimens. Renal complications not related to MM progression were observed in 19 (17%) patients; thrombotic microangiopathy (TMA) was seen in 6 (5%) patients, albuminuria >1 gr/day in 7 patients (6%) and at least grade 3 acute kidney injury (AKI) which could not be otherwise explained in 6 patients (5%). A total of 15 patients discontinued CFZ and dosing was reinitiated at a lower level in one patient with AKI. Albuminuria was associated with focal segmental glomerulosclerosis in the renal biopsy (performed in a total of 6 patients). Renal complications during CFZ therapy are common, occur mostly early and are unpredictable. A potential effect of CFZ on the renal endothelium could be implicated in the pathogenesis of these complications and may also share common pathophysiology with cardiovascular effects of CFZ.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Syndromes of thrombotic microangiopathy.
- Record: found
- Abstract: found
- Article: not found
Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma.
- Record: found
- Abstract: not found
- Article: not found