- Record: found
- Abstract: found
- Article: found
Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice

Read this article at
Abstract
Mesenchymal stem cell-derived exosomes (MSC-Exo) have robust anti-inflammatory effects in the treatment of neurological diseases such as epilepsy, stroke, or traumatic brain injury. While astrocytes are thought to be mediators of these effects, their precise role remains poorly understood. To address this issue, we investigated the putative therapeutic effects and mechanism of MSC-Exo on inflammation-induced alterations in astrocytes.
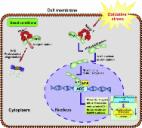
Methods: Lipopolysaccharide (LPS)-stimulated hippocampal astrocytes in primary culture were treated with MSC-Exo, which were also administered in pilocarpine-induced status epilepticus (SE) mice. Exosomal integration, reactive astrogliosis, inflammatory responses, calcium signaling, and mitochondrial membrane potentials (MMP) were monitored. To experimentally probe the molecular mechanism of MSC-Exo actions on the inflammation-induced astrocytic activation, we inhibited the nuclear factor erythroid-derived 2, like 2 (Nrf2, a key mediator in neuroinflammation and oxidative stress) by sgRNA (in vitro) or ML385 (Nrf2 inhibitor) in vivo.
Results: MSC-Exo were incorporated into hippocampal astrocytes as well as attenuated reactive astrogliosis and inflammatory responses in vitro and in vivo. Also, MSC-Exo ameliorated LPS-induced aberrant calcium signaling and mitochondrial dysfunction in culture, and SE-induced learning and memory impairments in mice. Furthermore, the putative therapeutic effects of MSC-Exo on inflammation-induced astrocytic activation (e.g., reduced reactive astrogliosis, NF-κB deactivation) were weakened by Nrf2 inhibition.
Conclusions: Our results show that MSC-Exo ameliorate inflammation-induced astrocyte alterations and that the Nrf2-NF-κB signaling pathway is involved in regulating astrocyte activation in mice. These data suggest the promising potential of MSC-Exo as a nanotherapeutic agent for the treatment of neurological diseases with hippocampal astrocyte alterations.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus.
- Record: found
- Abstract: found
- Article: not found
An astrocytic basis of epilepsy.
- Record: found
- Abstract: found
- Article: found