- Record: found
- Abstract: found
- Article: found
International Consensus (ICON): allergic reactions to vaccines
Read this article at
Abstract
Background
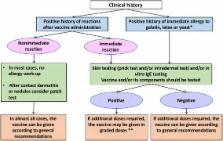
Routine immunization, one of the most effective public health interventions, has effectively reduced death and morbidity due to a variety of infectious diseases. However, allergic reactions to vaccines occur very rarely and can be life threatening. Given the large numbers of vaccines administered worldwide, there is a need for an international consensus regarding the evaluation and management of allergic reactions to vaccines.
Methods
Following a review of the literature, and with the active participation of representatives from the World Allergy Organization (WAO), the European Academy of Allergy and Clinical Immunology (EAACI), the American Academy of Allergy, Asthma, and Immunology (AAAAI), and the American College of Allergy, Asthma, and Immunology (ACAAI), the final committee was formed with the purpose of having members who represented a wide-range of countries, had previously worked on vaccine safety, and included both allergist/immunologists as well as vaccinologists.
Results
Consensus was reached on a variety of topics, including: definition of immediate allergic reactions, including anaphylaxis, approaches to distinguish association from causality, approaches to patients with a history of an allergic reaction to a previous vaccine, and approaches to patients with a history of an allergic reaction to components of vaccines.
Conclusions
This document provides comprehensive and internationally accepted guidelines and access to on-line documents to help practitioners around the world identify allergic reactions following immunization. It also provides a framework for the evaluation and further management of patients who present either following an allergic reaction to a vaccine or with a history of allergy to a component of vaccines.
Related collections
Most cited references133
- Record: found
- Abstract: found
- Article: not found
The diagnosis and management of anaphylaxis practice parameter: 2010 update.
- Record: found
- Abstract: found
- Article: not found
A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force.
- Record: found
- Abstract: found
- Article: not found