- Record: found
- Abstract: found
- Article: found
Growth factor restriction impedes progression of wound healing following cataract surgery: identification of VEGF as a putative therapeutic target
Read this article at
Abstract
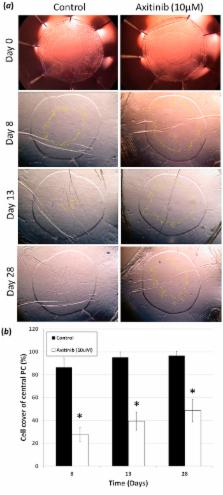
Secondary visual loss occurs in millions of patients due to a wound-healing response, known as posterior capsule opacification (PCO), following cataract surgery. An intraocular lens (IOL) is implanted into residual lens tissue, known as the capsular bag, following cataract removal. Standard IOLs allow the anterior and posterior capsules to become physically connected. This places pressure on the IOL and improves contact with the underlying posterior capsule. New open bag IOL designs separate the anterior capsule and posterior capsules and further reduce PCO incidence. It is hypothesised that this results from reduced cytokine availability due to greater irrigation of the bag. We therefore explored the role of growth factor restriction on PCO using human lens cell and tissue culture models. We demonstrate that cytokine dilution, by increasing medium volume, significantly reduced cell coverage in both closed and open capsular bag models. This coincided with reduced cell density and myofibroblast formation. A screen of 27 cytokines identified nine candidates whose expression profile correlated with growth. In particular, VEGF was found to regulate cell survival, growth and myofibroblast formation. VEGF provides a therapeutic target to further manage PCO development and will yield best results when used in conjunction with open bag IOL designs.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration.
- Record: found
- Abstract: found
- Article: not found