- Record: found
- Abstract: found
- Article: found
The role of the brown adipose tissue in β3-adrenergic receptor activation-induced sleep, metabolic and feeding responses
Read this article at
Abstract
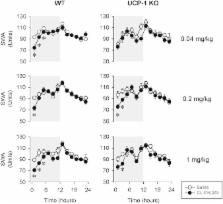
Brown adipose tissue (BAT) is regulated by the sympathetic nervous system via β3-adrenergic receptors (β3-AR). Here we tested the hypothesis that pharmacological stimulation of β3-ARs leads to increased sleep in mice and if this change is BAT dependent. In wild-type (WT) animals, administration of CL-316,243, a selective β3-AR agonist, induced significant increases in non-rapid-eye movement sleep (NREMS) lasting for 4–10 h. Simultaneously, electroencephalographic slow-wave activity (SWA) was significantly decreased and body temperature was increased with a delay of 5–6 h. In uncoupling protein 1 (UCP-1) knockout mice, the middle and highest doses of the β3-AR agonist increased sleep and suppressed SWA, however, these effects were significantly attenuated and shorter-lasting as compared to WT animals. To determine if somnogenic signals arising from BAT in response to β3-AR stimulation are mediated by the sensory afferents of BAT, we tested the effects of CL-316,243 in mice with the chemical deafferentation of the intra-scapular BAT pads. Sleep responses to CL-316,243 were attenuated by ~50% in intra-BAT capsaicin-treated mice. Present findings indicate that the activation of BAT via β3-AR leads to increased sleep in mice and that this effect is dependent on the presence of UCP-1 protein and sleep responses require the intact sensory innervation of BAT.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid.
- Record: found
- Abstract: found
- Article: not found
Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats.
- Record: found
- Abstract: found
- Article: not found
