- Record: found
- Abstract: found
- Article: found
Feasibility of repairing skin defects by VEGF 165 gene‐modified iPS‐HFSCs seeded on a 3D printed scaffold containing astragalus polysaccharide
Read this article at
Abstract
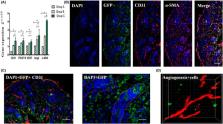
The preparation of biodegradable scaffolds loaded with cells and cytokine is a feature of tissue‐engineered skin. IPSCs‐based tissue‐engineered skin treatment for wound repair is worth exploring. Healthy human skin fibroblasts were collected and reprogrammed into iPSCs. After gene modification and induction, CK19 +/Integrinβ1 +/CD200 + VEGF 165 gene‐modified iPS‐HFSCs GFP were obtained and identified by a combination of immunofluorescence and RT‐qPCR. Astragalus polysaccharide‐containing 3D printed degradable scaffolds were prepared and co‐cultured with VEGF 165 gene‐modified iPS‐HFSCs GFP, and the biocompatibility and spatial structure of the tissue‐engineered skin was analysed by cell counting kit‐8 (CCK8) assay and scanning electron microscopy. Finally, the tissue‐engineered skin was transplanted onto the dorsal trauma of nude mice, and the effect of tissue‐engineered skin on the regenerative repair of total skin defects was evaluated by a combination of histology, immunohistochemistry, immunofluorescence, RT‐qPCR, and in vivo three‐dimensional reconstruction under two‐photon microscopy. CK19 +/Integrinβ1 +/CD200 + VEGF 165 gene‐modified iPS‐HFSCs GFP, close to the morphology and phenotype of human‐derived hair follicle stem cells, were obtained. The surface of the prepared 3D printed degradable scaffold containing 200 μg/mL astragalus polysaccharide was enriched with honeycomb‐like meshwork, which was more conducive to the proliferation of the resulting cells. After tissue‐engineered skin transplantation, combined assays showed that it promoted early vascularization, collagen and hair follicle regeneration and accelerated wound repair. VEGF 165 gene‐modified iPS‐HFSCs GFP compounded with 3D printed degradable scaffolds containing 200 μg/mL astragalus polysaccharide can directly and indirectly participate in vascular, collagen, and hair follicle regeneration in the skin, achieving more complete structural and functional skin regenerative repair.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Microbiota and maintenance of skin barrier function
- Record: found
- Abstract: found
- Article: not found
Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells
- Record: found
- Abstract: not found
- Article: not found