- Record: found
- Abstract: found
- Article: found
Microarray-based screening system identifies temperature-controlled activity of Connexin 26 that is distorted by mutations
Read this article at
Abstract
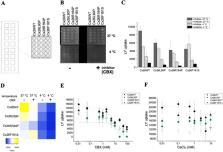
Here, we show that human Connexin 26 (hCx26 or Cx26WT) hemichannel opening rapidly enables the transport of small molecules when triggered by temperature and by compensation of the Ca 2+ blockade with EDTA. Point mutations within Cx26 were analysed by a novel optical microarray-based Lucifer Yellow uptake assay or by two electrode voltage clamp (TEVC) on frog oocytes to monitor simultaneous activities of channel proteins. Point mutations L90P, F161S, R184P or K188N influenced the temperature-dependent activity drastically. Since several mutations blocked trafficking, the temperature-dependent activity of the recombinant synthesized and purified wild-type Cx26WT and Cx26K188N hemichannel was tested by liposome flux assay (LFA) and on a microarray-based Lucifer Yellow uptake assay under warm conditions (>30 °C). The data from TEVC measurements and dye flux experiments showed that the mutations gave no or only a weak activity at increased temperature (>30 °C). We conclude that the position K188 in the Cx26WT forms a temperature-sensitive salt bridge with E47 whereas the exchange to K188N destabilizes the network loop- gating filter, which was recently identified as a part of the flexible Ca 2+ binding site. We assume that the temperature sensitivity of Cx26 is required to protect cells from uncontrolled release or uptake activities through Cx26 hemichannels.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Gap junctions: structure and function (Review).

- Record: found
- Abstract: found
- Article: found
Simple and efficient site-directed mutagenesis using two single-primer reactions in parallel to generate mutants for protein structure-function studies
- Record: found
- Abstract: found
- Article: not found