- Record: found
- Abstract: found
- Article: found
Extracellular resistance is sensitive to tissue sodium status; implications for bioimpedance-derived fluid volume parameters in chronic kidney disease
Read this article at
Abstract
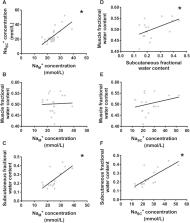
Multifrequency bioimpedance spectroscopy (BIS) is an established method for assessing fluid status in chronic kidney disease (CKD). However, the technique is lacking in predictive value and accuracy. BIS algorithms assume constant tissue resistivity, which may vary with changing tissue ionic sodium concentration (Na +). This may introduce significant inaccuracies to BIS outputs. To investigate this, we used 23Na magnetic resonance imaging (MRI) to measure Na + in muscle and subcutaneous tissues of 10 healthy controls (HC) and 20 patients with CKD 5 (not on dialysis). The extracellular (Re) and intracellular (Ri) resistance, tissue capacitance, extracellular (ECW) and total body water (TBW) were measured using BIS. Tissue water content was assessed using proton density-weighted MRI with fat suppression. BIS-derived volume indices were comparable in the two groups (OH: HC − 0.4 ± 0.9 L vs. CKD 0.5 ± 1.9 L, p = 0.13). However, CKD patients had higher Na + (HC 21.2 ± 3.0, CKD 25.3 ± 7.4 mmol/L; p = 0.04) and significantly lower Re (HC 693 ± 93.6, CKD 609 ± 74.3 Ohms; p = 0.01); Ri and capacitance did not vary. Na + showed a significant inverse linear relationship to Re (r s = − 0.598, p < 0.01) but not Ri. This relationship of Re (y) and Na + (x) is described through equation y = − 7.39x + 814. A 20% increase in tissue ionic Na + is likely to overestimate ECW by 1.2–2.4L. Tissue Na + concentration has a significant inverse linear relationship to Re. BIS algorithms to account for this effect could improve prediction accuracy of bioimpedance derived fluid status in CKD.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial.
- Record: found
- Abstract: found
- Article: not found
Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin.
- Record: found
- Abstract: found
- Article: not found