- Record: found
- Abstract: found
- Article: found
Efflux transporters in blood-brain interfaces of the developing brain
Read this article at
Abstract
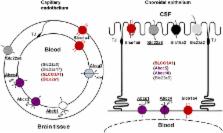
The cerebral microvessel endothelium forming the blood-brain barrier (BBB) and the epithelium of the choroid plexuses forming the blood-CSF barrier (BCSFB) operate as gatekeepers for the central nervous system. Exposure of the vulnerable developing brain to chemical insults can have dramatic consequences for brain maturation and lead to life-long neurological diseases. The ability of blood-brain interfaces to efficiently protect the immature brain is therefore an important pathophysiological issue. This is also key to our understanding of drug entry into the brain of neonatal and pediatric patients. Non-specific paracellular diffusion through barriers is restricted early during development, but other neuroprotective properties of these interfaces differ between the developing and adult brains. This review focuses on the developmental expression and function of various classes of efflux transporters. These include the multispecific transporters of the ATP-binding cassette transporter families ABCB, ABCC, ABCG, the organic anion and cation transporters of the solute carrier families SLC21/SLCO and SLC22, and the peptide transporters of the SLC15 family. These transporters play a key role in preventing brain entry of blood-borne molecules such as drugs, environmental toxicants, and endogenous metabolites, or else in increasing the clearance of potentially harmful organic ions from the brain. The limited data available for laboratory animals and human highlight transporter-specific developmental patterns of expression and function, which differ between blood-brain interfaces. The BCSFB achieves an adult phenotype earlier than BBB. Efflux transporters at the BBB appear to be regulated by various factors subsequently secreted by neural progenitors and astrocytes during development. Their expression is also modulated by oxidative stress, inflammation, and exposure to xenobiotic inducers. A better understanding of these regulatory pathways during development, in particular the signaling pathways triggered by oxidative stress and xenobiotics, may open new opportunities to therapeutic manipulation in view to improve or restore neuroprotective functions of the blood-brain interfaces in the context of perinatal injuries.
Related collections
Most cited references78
- Record: found
- Abstract: found
- Article: not found
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview.
- Record: found
- Abstract: found
- Article: not found
Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense.

- Record: found
- Abstract: found
- Article: found