- Record: found
- Abstract: found
- Article: found
Adjuvant radiotherapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials
Read this article at
Abstract
Background
Radiotherapy (RT) improves overall survival (OS) of breast cancer patients after breast conserving surgery and after mastectomy in patients with involved lymph nodes (LN). The contribution of RT to the regional LN to this survival benefit was poorly understood. Recently, the results of three large randomized trials addressing this question have become available.
Material and methods
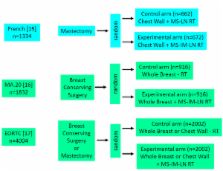
The published abstracts (full publication pending) of the MA.20 (n=1832) and the EORTC 22922–10925 (EORTC) (n=4004) trial and the full publication of the French trial (n=1334) were basis of the meta-analysis. Main eligibility criteria were positive axillary LN (all trials), LN negative disease with high risk for recurrence (MA.20), and medial/central tumor location (French, EORTC). The MA.20 and the EORTC trial tested the effect of additional regional RT to the internal mammary (IM) LN and medial supraclavicular (MS) LN, whereas in the French trial all patients received RT to the MS-LN and solely RT to the IM-LN was randomized. Primary endpoint was OS. Secondary endpoints were disease-free survival (DFS) and distant metastasis free survival (DMFS).
Results
Regional RT of the MS-LN and the IM-LN (MA.20 and EORTC) resulted in a significant improvement of OS (Hazard Ratio (HR) 0.85 (95% CL 0.75 - 0.96)). Adding the results of the French trial and using the random effects model to respect the different design of the French trial, the effect on OS of regional radiotherapy was still significant (HR 0.88 (95% CL 0.80 - 0.97)). The absolute benefits in OS were 1.6% in the MA.20 trial at 5 years, 1.6% in the EORTC trial at 10 years, and 3.3% in the French trial at 10 years (not significant in single trials). Regional radiotherapy of the MS-LN and the IM-LN (MA.20 and EORTC) was associated with a significant improvement of DFS (HR 0.85 (95% CL 0.77 - 0.94)) and DMFS (HR 0.82 (95% CL 0.73 - 0.92)). The effect sizes were not significantly different between trials for any end point.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints.
- Record: found
- Abstract: found
- Article: not found
Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial.
- Record: found
- Abstract: found
- Article: not found