- Record: found
- Abstract: found
- Article: not found
Improved in Vitro Blood Compatibility of Polycaprolactone Nanowire Surfaces
Read this article at
Abstract
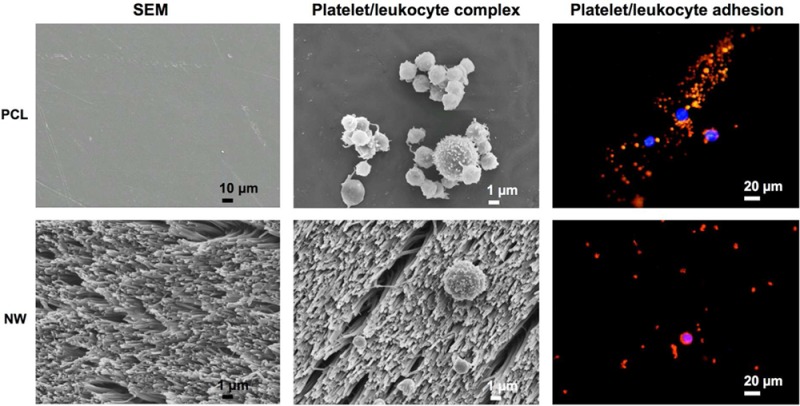
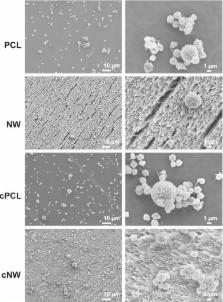
There are a multitude of polymeric materials currently utilized to prepare a variety of blood-contacting implantable medical devices. These devices include tissue grafts, coronary artery and vascular stents, and orthopedic implants. The thrombogenic nature of such materials can cause serious complications in patients, and ultimately lead to functional failure. To date, there is no truly hemocompatible biomaterial surface. Nanostructured surfaces improve cellular interactions but there is a limited amount of information regarding their blood compatibility. In this study, the in vitro blood compatibility of four different surfaces (control, PCL; nanowire, NW; collagen immobilized control, cPCL; collagen immobilized nanowire, cNW) were investigated for their use as interfaces for blood-contacting implants. The results presented here indicate enhanced in vitro blood compatibility of nanowire surfaces compared control surfaces. Although there were no significant differences in leukocyte adhesion, there was a decrease in platelet adhesion on NW surfaces. Scanning electron microscopy images showed a decrease in platelet/leukocyte complexes on cNW surfaces and no apparent complexes were formed on NW surfaces compared to PCL and cPCL surfaces. The increase in these complexes likely contributed to a higher expression of specific markers for platelet and leukocyte activation on PCL and cPCL surfaces. No significant differences were found in contact and complement activation on any surface. Further, thrombin antithrombin complexes were significantly reduced on NW surfaces. A significant increase in hemolysis and fibrinogen adsorption was identified on PCL surfaces likely caused by its hydrophobic surface. This work shows the improved blood-compatibility of nanostructured surfaces, identifying this specific nanoarchitecture as a potential interface for promoting the long-term success of blood-contacting biomaterials.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Synthetic biodegradable polymers as orthopedic devices
- Record: found
- Abstract: found
- Article: not found
Interpretation of protein adsorption: surface-induced conformational changes.
- Record: found
- Abstract: not found
- Article: not found
