- Record: found
- Abstract: found
- Article: found
Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens
Read this article at
Abstract
Background:
Chronic ethanol exposure reduces dopamine transmission in the nucleus accumbens, which may contribute to the negative affective symptoms associated with ethanol withdrawal. Kappa opioid receptors have been implicated in withdrawal-induced excessive drinking and anxiety-like behaviors and are known to inhibit dopamine release in the nucleus accumbens. The effects of chronic ethanol exposure on kappa opioid receptor-mediated changes in dopamine transmission at the level of the dopamine terminal and withdrawal-related behaviors were examined.
Methods:
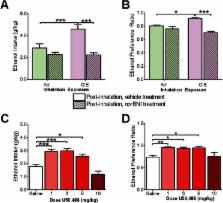
Five weeks of chronic intermittent ethanol exposure in male C57BL/6 mice were used to examine the role of kappa opioid receptors in chronic ethanol-induced increases in ethanol intake and marble burying, a measure of anxiety/compulsive-like behavior. Drinking and marble burying were evaluated before and after chronic intermittent ethanol exposure, with and without kappa opioid receptor blockade by nor-binaltorphimine (10mg/kg i.p.). Functional alterations in kappa opioid receptors were assessed using fast scan cyclic voltammetry in brain slices containing the nucleus accumbens.
Results:
Chronic intermittent ethanol-exposed mice showed increased ethanol drinking and marble burying compared with controls, which was attenuated with kappa opioid receptor blockade. Chronic intermittent ethanol-induced increases in behavior were replicated with kappa opioid receptor activation in naïve mice. Fast scan cyclic voltammetry revealed that chronic intermittent ethanol reduced accumbal dopamine release and increased uptake rates, promoting a hypodopaminergic state of this region. Kappa opioid receptor activation with U50,488H concentration-dependently decreased dopamine release in both groups; however, this effect was greater in chronic intermittent ethanol-treated mice, indicating kappa opioid receptor supersensitivity in this group.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway.

- Record: found
- Abstract: found
- Article: found
Addiction is a Reward Deficit and Stress Surfeit Disorder
- Record: found
- Abstract: found
- Article: not found