- Record: found
- Abstract: found
- Article: found
Expression of Somatostatin Receptor 2 in Somatotropinoma Correlated with the Short-Term Efficacy of Somatostatin Analogues
Read this article at
Abstract
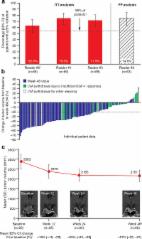
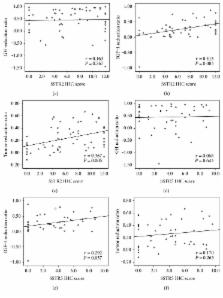
The expression of somatostatin receptor subtypes (SSTRs) in pituitary growth hormone- (GH-) secreting adenomas may predict the response to somatostatin analogues (SSA). Our aim was to evaluate the value of the immunohistochemical (IHC) scores of 2 subtypes, SSTR2 and SSTR5, in predicting the short-term efficacy of SSA therapy in patients with active acromegaly. Ninety-three newly diagnosed acromegalic patients were included in our study. These patients were categorized into either a SSA-pretreated group (SA, n = 63) or a direct-surgery group (DS, n = 30), depending on whether or not presurgical SSA treatment was received. IHC analysis, using a 12-grade scoring system, with rabbit monoclonal antibodies against SSTR2 and SSTR5, was performed on all adenoma tissues. The reduction of GH, IGF-1, and tumor size after treatment with SSA for 3 months was measured. Compared with that in the DS group, SSTR2 expression was lower in the SA group. Additionally, in the SA group, SSTR2 expression was positively correlated with the reduction of IGF-1 and tumor volume. However, there was no correlation between the SSTR5 score and the efficacy of SSA. In conclusion, the protein expression of SSTR2, but not of SSTR5, is a valuable indicator in predicting biochemical and tumor size response to short-term SSA treatment in acromegalic patients.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Acromegaly pathogenesis and treatment.
- Record: found
- Abstract: found
- Article: not found