- Record: found
- Abstract: found
- Article: found
Developing an experimental necrotic enteritis model in turkeys - the impact of Clostridium perfringens, Eimeria meleagrimitis and host age on frequency of severe intestinal lesions
Read this article at
Abstract
Background
Necrotic enteritis is a significant problem to the poultry industry globally and, in Norway up to 30% of Norwegian turkey grow-outs can be affected. However, despite an awareness that differences exist between necrotic enteritis in chickens and turkeys, little information exists concerning the pathogenesis, immunity, microbiota or experimental reproduction of necrotic enteritis in turkeys. In particular, it is important to determine the appearance of the gross lesions, the age dependency of the disease and the role of netB toxin of Clostridium perfringens. To this end, we report our findings in developing an in vivo experimental model of necrotic enteritis in turkeys.
Results
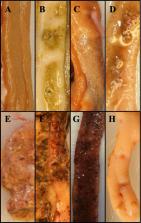
A four tier (0–3) scoring system with clearly defined degrees of severity of macroscopic intestinal lesions was developed, based on 2312 photographic images of opened intestines from 810 B.U.T. 10 or B.U.T. Premium turkeys examined in nine experiments. Loss of macroscopically recognizable villi in the anterior small intestine was established as the defining lesion qualifying for a score 3 (severe intestinal lesions). The developed scoring system was used to identify important factors in promoting high frequencies of turkeys with severe lesions: a combined Eimeria meleagrimitis and Clostridium perfringens challenge, challenge at five rather than 3 weeks of age, the use of an Eimeria meleagrimitis dose level of at least 5000 oocysts per bird and finally, examination of the intestines of 5-week-old turkeys at 125 to 145 h after Eimeria meleagrimitis inoculation. Numbers of oocysts excreted were not influenced by Clostridium perfringens inoculation or turkey age. Among three different lesion score outcomes tested, frequency of severe lesions proved superior in discriminating between impact of four combinations of Clostridium perfringens inoculation and turkey age at challenge.
Related collections
Most cited references16

- Record: found
- Abstract: found
- Article: found
The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review
- Record: found
- Abstract: found
- Article: not found