- Record: found
- Abstract: found
- Article: found
Health research system resilience: lesson learned from the COVID-19 crisis
Read this article at
Abstract
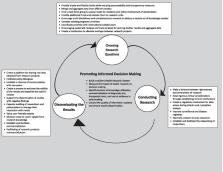
Producing evidence in epidemics is crucial to control the current epidemic and prevent its recurrence in the future. Data must be collected and analyzed rapidly to recognize the most efficient and feasible methods with proper timelines. However, there are many challenges a research system may encounter during a crisis. This article has presented lessons learned from the COVID-19 pandemic for health research system (HRS) to deal with current and future crises. Therefore, a HRS needs to produce and use evidence in such a situation. The components Knowledge Translation Self-Assessment Tool for Research Institutes (SATORI) framework was used to review the actions required and respond to the COVID-19 pandemic in a national HRS. This framework consists of four categories of defining the research question, conducting research, translating the research results, and promoting the use of evidence. The work is proposed actions in response to the COVID-19 crisis and improving a HRS's resilience. While COVID-19 has serious harm to the health and broader socio-economic consequences, this threat should be accounted for as an opportunity to make research systems more accountable and responsible in the timely production and utilization of knowledge. It is time to seriously think about how HRS can build a better back to be resilient to potential shock and prepare for unforeseen emerging conditions.
Related collections
Most cited references12
- Record: found
- Abstract: not found
- Article: not found
Fair Allocation of Scarce Medical Resources in the Time of Covid-19
- Record: found
- Abstract: not found
- Article: not found
Waste in covid-19 research.

- Record: found
- Abstract: found
- Article: found