- Record: found
- Abstract: found
- Article: found
Randomised clinical trial: a leucine‐metformin‐sildenafil combination (NS‐0200) vs placebo in patients with non‐alcoholic fatty liver disease
Read this article at
Summary
Background
Sirtuin 1 (Sirt1) is suppressed in non‐alcoholic fatty liver disease ( NAFLD), while its’ stimulation or overexpression results in reduced disease severity in pre‐clinical NAFLD models. Leucine allosterically activates Sirt1 and synergise with other Sirt/ AMPK/ NO pathway activators. We developed a triple combination of leucine, metformin and sildenafil ( NS‐0200), which was effective in a mouse model of non‐alcoholic steatohepatitis ( NASH).
Aim
To report the results from a Phase 2, randomised clinical trial of of NS‐0200 in 91 subjects with NAFLD (liver fat ≥15% by magnetic resonance imaging‐proton‐density fat fraction ( MRI‐ PDFF)).
Methods
Subjects were randomised to placebo, low‐dose (1.1 g leucine/0.5 g metformin/0.5 mg sildenafil) or high‐dose NS‐0200 (1.1 g leucine/0.5 g metformin/1.0 mg sildenafil) b.d. for 16 weeks; change in hepatic fat was assessed via MRI‐ PDFF, and lipid metabolism was assessed via changes in the lipidomic signature. Seventy subjects completed the trial and met a priori compliance criteria. Analyses were conducted on the full cohort and on those with alanine aminotransferase (ALT) values above median (50 U/L; n = 35).
Results
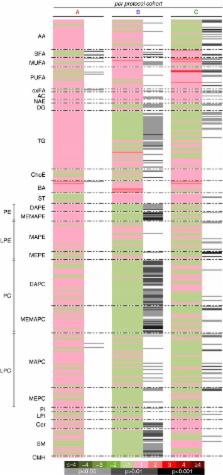
In the full cohort, active treatments did not separate from placebo. High dose NS‐0200 reduced hepatic fat by 15.7% (relative change from baseline) in the high ALT group ( P < 0.005) while low dose NS‐0200 and placebo did not significantly change hepatic fat. Lipidomic analysis showed dose‐responsive treatment effects in both overall and high ALT cohorts, with significant decreases in metabolically active lipids and up‐regulation of fatty acid oxidation.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation.
- Record: found
- Abstract: found
- Article: not found
Sirt1 protects against high-fat diet-induced metabolic damage.
- Record: found
- Abstract: found
- Article: not found