- Record: found
- Abstract: found
- Article: not found
The synthetic and therapeutic expedition of isoxazole and its analogs
Read this article at
Abstract
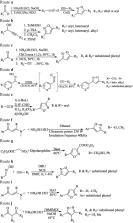
Isoxazole, constituting an important family of five-membered heterocycles with one oxygen atom and one nitrogen atom at adjacent positions is of immense importance because of its wide spectrum of biological activities and therapeutic potential. It is, therefore, of prime importance that the development of new synthetic strategies and designing of new isoxazole derivatives should be based on the most recent knowledge emerging from the latest research. This review is an endeavor to highlight the progress in the chemistry and biological activity of isoxazole derivatives which could provide a low-height flying bird’s eye view of isoxazole derivatives to the medicinal chemists for the development of clinically viable drugs using this information.
Related collections
Most cited references172
- Record: found
- Abstract: not found
- Article: not found
Isoxazole ring as a useful scaffold in a search for new therapeutic agents
- Record: found
- Abstract: found
- Article: not found
Development of Pyrazolone and Isoxazol-5-one Cambinol Analogues as Sirtuin Inhibitors
- Record: found
- Abstract: found
- Article: not found
