- Record: found
- Abstract: found
- Article: found
Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation

Read this article at
Abstract
Background
Stimulation of the vagus nerve has modulating, anti-inflammatory effects on the cellular immune response in the blood and the spleen, stabilizing brain function. Here, we aimed to investigate its potential effects on immune-to-brain communication focusing on neurophysiological readouts and leukocyte migration to the brain during severe sepsis-like endotoxemia.
Methods
Systemic inflammation was induced by intravenous administration of lipopolysaccharide (LPS; 5 mg/kg). Animals received either no manipulation of the vagus nerve, vagotomy, or vagotomy plus vagus nerve stimulation of the distal trunk. Somatosensory evoked potentials and evoked flow velocity response were measured for 4.5 h as indicators of brain function and neurovascular coupling, respectively. In addition, brain areas with (cortex) and without (hypothalamus) tight blood-brain barrier were studied separately using immunohistochemistry and RT-PCR. Moreover, plasma cytokine and leptin levels were analyzed by ELISA.
Results
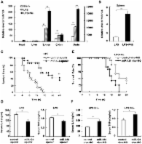
LPS induced a decline of both neurophysiological parameters, which was prevented by vagus nerve stimulation. As for peripheral organs, LPS-stimulated neutrophil counts increased in the brain and colocalized in the brain with endothelial intercellular adhesion molecule (ICAM)-1. Interestingly, vagal stimulation reduced this colocalization and decreased nuclear translocation of the brain cell activation marker nuclear factor interleukin 6 (NF-IL6). Furthermore, it reduced the gene expression of inflammatory markers and extravasation signals (IL-6, CXCL-1, ICAM-1) in the hypothalamus but not the cortex linked to a moderate decrease in circulating cytokine levels (interleukin 6, tumor necrosis factor alpha) as well as lower plasma leptin concentration.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
The cholinergic anti-inflammatory pathway: a critical review.
- Record: found
- Abstract: found
- Article: found
MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines
- Record: found
- Abstract: found
- Article: not found