- Record: found
- Abstract: found
- Article: found
Genetics of acute myeloid leukemia in the elderly: mutation spectrum and clinical impact in intensively treated patients aged 75 years or older
Read this article at
Abstract
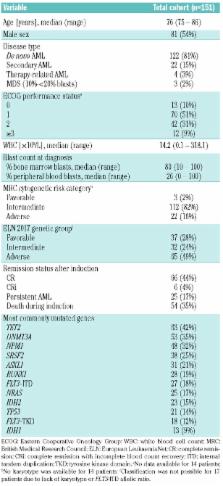
A cute myeloid leukemia is a disease of the elderly (median age at diagnosis, 65–70 years). The prognosis of older acute myeloid leukemia patients is generally poor. While genetic markers have become important tools for risk stratification and treatment selection in young and middle-aged patients, their applicability in very old patients is less clear. We sought to validate existing genetic risk classification systems and identify additional factors associated with outcomes in intensively treated patients aged ≥75 years. In 151 patients who received induction chemotherapy in the AMLCG-1999 trial, we investigated recurrently mutated genes using a targeted sequencing assay covering 64 genes. The median number of mutated genes per patient was four. The most commonly mutated genes were TET2 (42%), DNMT3A (35%), NPM1 (32%), SRSF2 (25%) and ASXL1 (21%). The complete remission rate was 44% and the 3-year survival was 21% for the entire cohort. While adverse-risk cytogenetics (MRC classification) were associated with shorter overall survival ( P=0.001), NPM1 and FLT3-ITD mutations (present in 18%) did not have a significant impact on overall survival. Notably, none of the 13 IDH1-mutated patients (9%) reached complete remission. Consequently, the overall survival of this subgroup was significantly shorter than that of IDH1-wildtype patients ( P<0.001). In summary, even among very old, intensively treated, acute myeloid leukemia patients, adverse-risk cytogenetics predict inferior survival. The spectrum and relevance of driver gene mutations in elderly patients differs from that in younger patients. Our data implicate IDH1 mutations as a novel marker for chemorefractory disease and inferior prognosis. (AMLCG-1999 trial: clinicaltrials.gov identifier, NCT00266136)
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Prognostic relevance of integrated genetic profiling in acute myeloid leukemia.
- Record: found
- Abstract: found
- Article: not found
Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia.
- Record: found
- Abstract: found
- Article: not found