- Record: found
- Abstract: found
- Article: found
Water vs. cucurbituril rim: a fierce competition for guest solvation†
Read this article at
Abstract
Abstract
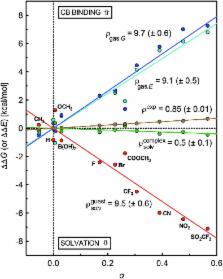
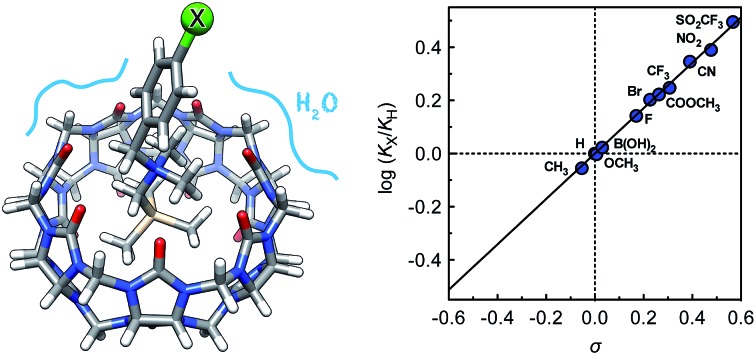
The impact of remote substituents on the affinity of cucurbit[ n]urils (CB[ n]) towards a homologous series of guests, which differ from one another only by a single substituent, and adopt the same geometry within the cavity of the macrocycle, is presented for the first time, and is used to decipher the competition between water and the carbonylated portal of CB[7] for the stabilization of positively charged guests. Binding affinities of CB[7] towards substituted N-benzyl-trimethylsilylmethylammonium cations relative to the unsubstituted member (X = H) range from 0.9 (X = CH 3) to 3.1 (X = SO 2CF 3), and correlate very precisely with a linear combination of Swain–Lupton field/inductive ( F; 67%) and resonance ( R; 33%) parameters tabulated for each substituent. We show that this subtle sensitivity results exclusively from the balance between two competing mechanisms, on which the substituents exert an approximately 11 times greater impact: (1) the solvation of the ammonium unit and its immediate surroundings by water in the free guests, and (2) the coulombic attraction between the ammonium unit and the rim of CB[7] in the complexes.
Related collections
Most cited references23
- Record: found
- Abstract: not found
- Article: not found
A survey of Hammett substituent constants and resonance and field parameters
- Record: found
- Abstract: found
- Article: not found
The cucurbit[n]uril family: prime components for self-sorting systems.
Author and article information
Notes
†Electronic supplementary information (ESI) available: Preparation and characterization of guests 3a–3k and their precursors; determination of binding affinities by competitive NMR titrations and ITC; computational procedures and data; Cartesian coordinates of the optimized structure of complexes 3a·CB[7] and 3e·CB[7]. See DOI: 10.1039/c5sc04475h