- Record: found
- Abstract: found
- Article: found
Mannitol induces selective astroglial death in the CA1 region of the rat hippocampus following status epilepticus
Read this article at
Abstract
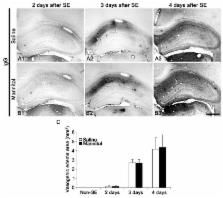
In the present study, we addressed the question of whether treatment with mannitol, an osmotic diuretic, affects astrogliovascular responses to status epilepticus (SE). In saline-treated animals, astrocytes exhibited reactive astrogliosis in the CA1-3 regions 2-4 days after SE. In the mannitol-treated animals, a large astroglial empty zone was observed in the CA1 region 2 days after SE. This astroglial loss was unrelated to vasogenic edema formation. There was no difference in SE-induced neuronal loss between saline- and mannitol-treated animals. Furthermore, mannitol treatment did not affect astroglial loss and vasogenic edema formation in the dentate gyrus and the piriform cortex. These findings suggest that mannitol treatment induces selective astroglial loss in the CA1 region independent of vasogenic edema formation following SE. These findings support the hypothesis that the susceptibility of astrocytes to SE is most likely due to the distinctive heterogeneity of astrocytes independent of hemodynamics. [BMB Reports 2015; 48(9): 507-512]
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Edema and brain trauma.

- Record: found
- Abstract: found
- Article: found
Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences
- Record: found
- Abstract: found
- Article: not found