- Record: found
- Abstract: found
- Article: found
Distribution and dynamics of electron transport complexes in cyanobacterial thylakoid membranes ☆
Read this article at
Abstract
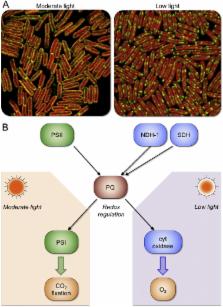
The cyanobacterial thylakoid membrane represents a system that can carry out both oxygenic photosynthesis and respiration simultaneously. The organization, interactions and mobility of components of these two electron transport pathways are indispensable to the biosynthesis of thylakoid membrane modules and the optimization of bioenergetic electron flow in response to environmental changes. These are of fundamental importance to the metabolic robustness and plasticity of cyanobacteria. This review summarizes our current knowledge about the distribution and dynamics of electron transport components in cyanobacterial thylakoid membranes. Global understanding of the principles that govern the dynamic regulation of electron transport pathways in nature will provide a framework for the design and synthetic engineering of new bioenergetic machinery to improve photosynthesis and biofuel production. This article is part of a Special Issue entitled: Organization and dynamics of bioenergetic systems in bacteria, edited by Conrad Mullineaux.
Highlights
-
•
Cyanobacterial thylakoid membranes carry out both oxygenic photosynthesis and respiration.
-
•
Electron transport components are located in the thylakoid membrane and functionally coordinate with each other.
-
•
Distribution and dynamics of electron transport components are physiologically regulated in response to environmental change.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis.
- Record: found
- Abstract: found
- Article: not found
A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria.
- Record: found
- Abstract: found
- Article: not found