- Record: found
- Abstract: found
- Article: found
The Association Between Hypertriglyceridemic-Waist Phenotype and Chronic Kidney Disease in Patients with Type 2 Diabetes: A Cross-Sectional METAL Study
Read this article at
Abstract
Background
The aim of this study was measuring the association between the hypertriglyceridemic-waist (HTGW) phenotype and chronic kidney disease in a large type 2 diabetes population.
Methods
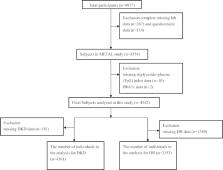
A total of 4254 diabetic patients from the cross-sectional Environmental Pollutant Exposure and Metabolic Diseases in Shanghai (METAL) study were enrolled. The hypertriglyceridemic-waist (HTGW) phenotype was defined as the presence of an elevated waist circumference (WC) and elevated triglyceride (TG) concentration. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m 2 or urinary albumin creatinine ratio (uACR) more than 30 mg/g. Linear and multiple logistic regression models were used for measuring the association between HTGW phenotype and chronic kidney disease.
Results
The prevalence of CKD was 29% and 35.8% in total participants and participants with HTGW phenotype, respectively. Subjects in the HTGW phenotype group were more likely to have CKD (OR 1.47, 95% CI: 1.11, 1.95) compared with subjects in the normal waist circumference and normal triglycerides (NTNW) group. HTGW phenotype was both associated with the increasing risk of decreased eGFR (OR 1.31, 95% CI: 1.02, 1.75) and elevated uACR (OR 1.57, 95% CI: 1.18, 2.11). Furthermore, the stratified analysis showed that the strongest positive association between HTGW phenotype and CKD presence was found in the subgroup of presence of hypertension. The associations were all fully adjusted for age, sex, BMI, current smoking, current drinking and other confounding factors.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man.

- Record: found
- Abstract: found
- Article: found
Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017
- Record: found
- Abstract: found
- Article: not found