- Record: found
- Abstract: found
- Article: not found
Decrypting Transition States by Light: Photoisomerization as a Mechanistic Tool in Brønsted Acid Catalysis
Read this article at
Abstract
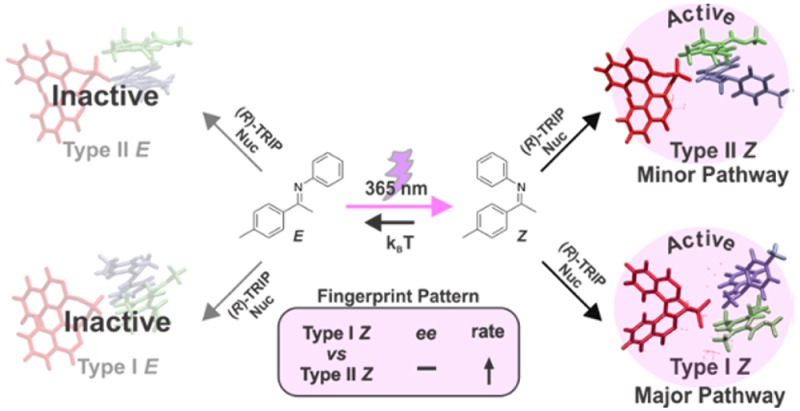
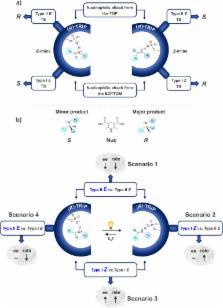
Despite the wide applicability of enantioselective Brønsted acid catalysis, experimental insight into transition states is very rare, and most of the mechanistic knowledge is gained by theoretical calculations. Here, we present an alternative approach (decrypting transition state by light = DTS- hν), which enables the decryption of the transition states involved in chiral phosphoric acids catalyzed addition of nucleophiles to imines. Photoisomerization of double bonds is employed as a mechanistic tool. For this class of reactions four pathways (Type I Z, Type I E, Type II Z, Type II E) are possible, leading to different enantiomers depending on the imine configuration ( E- or Z-imine) and on the nucleophilic attack site (top or bottom). We demonstrated that the imine double bond can be isomerized by light (365 nm LED) during the reaction leading to a characteristic fingerprint pattern of changes in reaction rate and enantioselectivity. This characteristic fingerprint pattern is directly correlated to the transition states involved in the transformation. Type I Z and Type II Z are demonstrated to be the competing pathways for the asymmetric transfer hydrogenation of ketimines, while in the nucleophilic addition of acetylacetone to N-Boc protected aldimines Type I E and Type II E are active. Accelerations on reaction rate up to 177% were observed for ketimines reduction. Our experimental findings are supported by quantum chemical calculations and noncovalent interaction analysis.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
NCIPLOT: a program for plotting non-covalent interaction regions.
- Record: found
- Abstract: not found
- Article: not found
