- Record: found
- Abstract: found
- Article: found
Product-oriented chemical surface modification of a levansucrase (SacB) via an ene-type reaction†
Read this article at
Abstract

Abstract
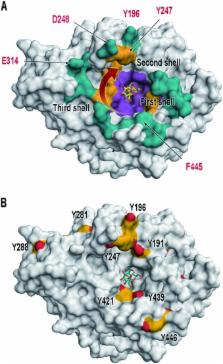
Carbohydrate processing enzymes are sophisticated tools of living systems that have evolved to execute specific reactions on sugars. Here we present for the first time the site-selective chemical modification of exposed tyrosine residues in SacB, a levansucrase from Bacillus megaterium ( Bm-LS) for enzyme engineering purposes via an ene-type reaction. Bm-LS is unable to sustain the synthesis of high molecular weight (HMW) levan (a fructose polymer) due to protein–oligosaccharide dissociation events occurring at an early stage during polymer elongation. We switched the catalyst from levan-like oligosaccharide synthesis to the efficient production of a HMW fructan polymer through the covalent addition of a flexible chemical side-chain that fluctuates over the central binding cavity of the enzyme preventing premature oligosaccharide disengagement.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Biocatalysis in organic chemistry and biotechnology: past, present, and future.
- Record: found
- Abstract: found
- Article: not found
Click chemistry generates privileged CH hydrogen-bonding triazoles: the latest addition to anion supramolecular chemistry.
Author and article information
Notes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc01244j