- Record: found
- Abstract: found
- Article: found
Pharmacologic therapy for engraftment arrhythmia induced by transplantation of human cardiomyocytes
Read this article at
Summary
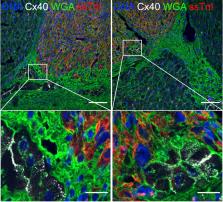
Heart failure remains a significant cause of morbidity and mortality following myocardial infarction. Cardiac remuscularization with transplantation of human pluripotent stem cell-derived cardiomyocytes is a promising preclinical therapy to restore function. Recent large animal data, however, have revealed a significant risk of engraftment arrhythmia (EA). Although transient, the risk posed by EA presents a barrier to clinical translation. We hypothesized that clinically approved antiarrhythmic drugs can prevent EA-related mortality as well as suppress tachycardia and arrhythmia burden. This study uses a porcine model to provide proof-of-concept evidence that a combination of amiodarone and ivabradine can effectively suppress EA. None of the nine treated subjects experienced the primary endpoint of cardiac death, unstable EA, or heart failure compared with five out of eight (62.5%) in the control cohort (hazard ratio = 0.00; 95% confidence interval: 0–0.297; p = 0.002). Pharmacologic treatment of EA may be a viable strategy to improve safety and allow further clinical development of cardiac remuscularization therapy.
Highlights
-
•
EA arises after hESC-CM transplantation in infarcted pigs
-
•
Combination pharmacotherapy prevents EA-related mortality and morbidity
-
•
Amiodarone and ivabradine significantly suppresses tachycardia and arrythmia burden
-
•
EA is polymorphic and may be due to interaction with intramural Purkinje fibers
Abstract
Potentially fatal engraftment arrhythmia (EA) arises after hESC-CM transplantation in infarcted pigs. Nakamura and colleagues present proof-of-concept evidence that a combination of amiodarone and ivabradine can effectively prevent EA-related mortality and suppresses tachycardia and arrhythmia burden. Thus, pharmacologic suppression of EA may be a viable strategy to improve safety and allow further clinical development of cardiac remuscularization therapy.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
The ImageJ ecosystem: An open platform for biomedical image analysis.
- Record: found
- Abstract: found
- Article: not found
hESC-Derived Cardiomyocytes Electrically Couple and Suppress Arrhythmias in Injured Hearts
- Record: found
- Abstract: found
- Article: not found