- Record: found
- Abstract: found
- Article: found
PICK1-Deficient Mice Exhibit Impaired Response to Cocaine and Dysregulated Dopamine Homeostasis
Read this article at
Abstract
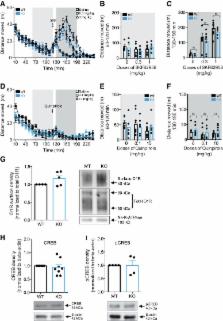
Protein interacting with C-kinase 1 (PICK1) is a widely expressed scaffold protein known to interact via its PSD-95/discs-large/ZO-1 (PDZ)-domain with several membrane proteins including the dopamine (DA) transporter (DAT), the primary target for cocaine’s reinforcing actions. Here, we establish the importance of PICK1 for behavioral effects observed after both acute and repeated administration of cocaine. In PICK1 knock-out (KO) mice, the acute locomotor response to a single injection of cocaine was markedly attenuated. Moreover, in support of a role for PICK1 in neuroadaptive changes induced by cocaine, we observed diminished cocaine intake in a self-administration paradigm. Reduced behavioral effects of cocaine were not associated with decreased striatal DAT distribution and most likely not caused by the ∼30% reduction in synaptosomal DA uptake observed in PICK1 KO mice. The PICK1 KO mice demonstrated preserved behavioral responses to DA receptor agonists supporting intact downstream DA receptor signaling. Unexpectedly, we found a prominent increase in striatal DA content and levels of striatal tyrosine hydroxylase (TH) in PICK1 KO mice. Chronoamperometric recordings showed enhanced DA release in PICK1 KO mice, consistent with increased striatal DA pools. Viral-mediated knock-down (KD) of PICK1 in cultured dopaminergic neurons increased TH expression, supporting a direct cellular effect of PICK1. In summary, in addition to demonstrating a key role of PICK1 in mediating behavioral effects of cocaine, our data reveal a so far unappreciated role of PICK1 in DA homeostasis that possibly involves negative regulation of striatal TH levels.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010.
- Record: found
- Abstract: found
- Article: not found
Scaffold proteins: hubs for controlling the flow of cellular information.
- Record: found
- Abstract: found
- Article: not found