- Record: found
- Abstract: found
- Article: found
Efficacy of preoperative chemotherapy regimens in patients with initially unresectable locally advanced gastric adenocarcinoma: capecitabine and oxaliplatin (XELOX) or with epirubicin (EOX)
Read this article at
Abstract
Purpose
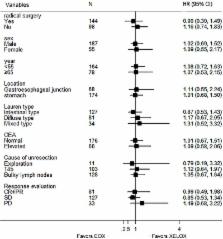
We assessed the effectiveness of EOX (capecitabine, oxaliplatin and epirubicin) compared with XELOX (capecitabine and oxaliplatin) as preoperative chemotherapy for initially unresectable locally advanced gastric cancer.
Methods
This is a prospective observational study. Patients with unresectable locally advanced gastric cancer were performed EOX regimen or XELOX regimen at the discretion of the investigators. They were assessed for response every 2 cycles by CT (computed tomography) scan. A multidisciplinary team reassessed resectability after 4 cycles. The primary endpoint was the response rate. Secondary end points included the R0 resection rate, survival and adverse events.
Results
From November 2008 to May 2015, 242 patients were enrolled; 112 of them were assigned to EOX regimen and 130 to XELOX regimen. The response rates were 33.0% and 33.8% respectively in EOX group and XELOX group ( P = 0.997). After 4 cycles of chemotherapy, 63 patients (56.3%) in EOX group and 81 patients (62.3%) in XELOX group received radical operation ( P = 0.408). There was no significant difference in progress-free survival (PFS, 12.0m vs. 15.4m, P = 0.925) and overall survival (OS, 25.7m vs. 29.0m, P = 0.783) in two groups. In addition, more adverse effects occurred in EOX group, such as more leucopenia (22.3% vs. 10.0%, P = 0.014), neutropenia (23.2% vs. 11.5%, P = 0.025), fatigue (11.6% vs. 3.8%, P = 0.041) and vomiting (10.7% vs. 2.3%, P = 0.015).
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry

- Record: found
- Abstract: found
- Article: not found