- Record: found
- Abstract: found
- Article: found
Comparison of Allogeneic Platelet-rich Plasma With Autologous Platelet-rich Plasma for the Treatment of Diabetic Lower Extremity Ulcers
Read this article at
Abstract
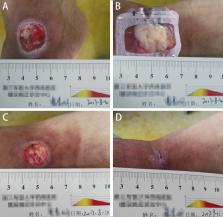
Autologous platelet-rich plasma (au-PRP) has been widely used for the management of refractory chronic wounds. However, patients with diabetic lower extremity ulcers (DLEUs) usually have complicated clinical conditions, and the utility of au-PRP is limited. In this study, the feasibility, effectiveness, and safety of allogeneic platelet-rich plasma (al-PRP) and au-PRP were investigated and compared in the treatment of DLEUs. A total of 75 in-patients with type 2 diabetes were assigned to the al-PRP group ( n = 20), au-PRP group ( n = 25), and conventional wound therapeutic (CWT) group ( n = 30) matched by the ankle brachial index and ulcer size from December 2015 to August 2018. Based on metabolic and nutritional regulation, infective control, and topical wound management, al-PRP, au-PRP, and CWT were administered to each group, respectively. Evaluation of treatment outcomes was determined by the parameters of wound healing and adverse reactions. The therapeutic times and average concentration of platelets were not significantly different between the au-PRP and al-PRP groups. The wound healing times of the al-PRP group (56.9 ± 29.22 d) and au-PRP group (55.6 ± 33.8 d) were significantly shorter than those of the CWT group (88.0 ± 43.4 d) ( P < 0.01), but there was no significant difference between the groups with PRP treatment. Although there was no significant difference in the daily healing area among all groups ( P > 0.05), the trend of the healing rate in the al-PRP group (16.77 ± 12.85 mm 2), au-PRP group (14.31 ± 18.28 mm 2), and CWT group (9.90 ± 8.51 mm 2) gradually decreased. No obvious adverse reactions (fever, edema, pain, skin itching, rash, or other sensory abnormalities) were observed in either the au-PRP or the al-PRP groups. Both al-PRP and au-PRP could effectively and safely promote wound healing in patients with DLEUs. Alternatively, al-PRP could be used for DLEUs as an off-the-shelf solution when au-PRP is limited.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial
- Record: found
- Abstract: found
- Article: not found