- Record: found
- Abstract: found
- Article: found
Analysis of the Bovine DLK1 Gene Polymorphism and Its Relation to Lipid Metabolism in Chinese Simmental
Read this article at
Abstract
Simple Summary
Delta-like non-canonical Notch ligand 1 (DLK1) is a candidate gene associated with lipid metabolism. In order to verify the function of the DLK1 gene on lipid metabolism in Chinese Simmental cattle, we identified the effect of DLK1 on lipid metabolism in bovine fetal fibroblast cells (BFFs). At the same time, we also detected the relationship between single-nucleotide polymorphism sites (SNPs) of bovine DLK1 with the economic traits and fatty acids composition in Chinese Simmental cattle, such as the carcass fat coverage rate, loin eye muscle area, and fat color score, etc. In the present study, we precisely constructed and transfected the overexpression and interference vectors of the DLK1 gene in BFFs. the results showed that the overexpression of the DLK1 gene could decrease the contents of intracellular triglycerides (TGs) while interference of the DLK1 gene could increase the TGs contents. Gas chromatography analysis of the fatty acid composition showed that the contents of octanoate acid and γ-linolenate acid were regulated as the expression of DLK1 was altered. We found two SNPs and genes associated with these traits in Chinese Simmental by the restriction fragment length polymorphism (RPLF-PCR) detection method. In summary, we verified the effects of bovine DLK1 on fatty acid metabolism from the cellular level to population genetic polymorphism, which also paved the way for further studying of the effects of the DLK1 gene on lipid metabolism in vivo.
Abstract
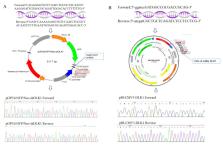
In this study, we precisely constructed and transfected the overexpression and interference vectors in BFFs to evaluate the role of DLK1 gene on lipid metabolism in vitro. The expression of of DLK1 in the mRNA and protein level tended to reduce, and TGs were significantly increased in the pGPU6-shDLK1 group compared to the control group ( p < 0.05). The expression of DLK1 in the mRNA and protein level were increased in the pBI-CMV3-DLK1 group compared to the control group, and the TGs content showed a significant decrease in the pBI-CMV3-DLK1 group ( p < 0.05). Meanwhile, we used the restriction fragment length polymorphism (RFLP-PCR) detection method to screen SNPs further to explore and analyze the relationship between the gene and the economic traits of 28-month-old Chinese Simmental and the fatty acids composition of cattle longissimus muscle. The result showed that two SNPs, IVS3 + 478 C > T and IVS3 + 609 T > G, were identified as being significantly associated with carcass and meat quality traits in Chinese Simmental, such as the carcass fat coverage rate, loin eye muscle area, and fat color score. In summary, our results indicated that DLK1 can affect lipid metabolism in bovine and these two SNPs might be applied as genetic markers of meat quality traits for beef cattle breeding.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Mechanisms of nutritional and hormonal regulation of lipogenesis.
- Record: found
- Abstract: found
- Article: not found
Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity.
- Record: found
- Abstract: found
- Article: not found