- Record: found
- Abstract: found
- Article: found
Tumor-Associated Macrophage Status in Cancer Treatment
Read this article at
Abstract
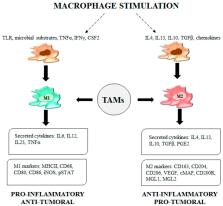
Tumor-associated macrophages (TAMs) represent the most abundant innate immune cells in tumors. TAMs, exhibiting anti-inflammatory phenotype, are key players in cancer progression, metastasis and resistance to therapy. A high TAM infiltration is generally associated with poor prognosis, but macrophages are highly plastic cells that can adopt either proinflammatory/antitumor or anti-inflammatory/protumor features in response to tumor microenvironment stimuli. In the context of cancer therapy, many anticancer therapeutics, apart from their direct effect on tumor cells, display different effects on TAM activation status and density. In this review, we aim to evaluate the indirect effects of anticancer therapies in the modulation of TAM phenotypes and pro/antitumor activity.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Macrophage regulation of tumor responses to anticancer therapies.
- Record: found
- Abstract: found
- Article: not found
Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients.
- Record: found
- Abstract: found
- Article: not found