- Record: found
- Abstract: found
- Article: found
Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells
Read this article at
Abstract
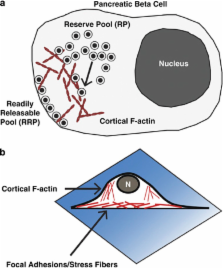
The maintenance of whole-body glucose homeostasis is critical for survival, and is controlled by the coordination of multiple organs and endocrine systems. Pancreatic islet β cells secrete insulin in response to nutrient stimuli, and insulin then travels through the circulation promoting glucose uptake into insulin-responsive tissues such as liver, skeletal muscle and adipose. Many of the genes identified in human genome-wide association studies of diabetic individuals are directly associated with β cell survival and function, giving credence to the idea that β-cell dysfunction is central to the development of type 2 diabetes. As such, investigations into the mechanisms by which β cells sense glucose and secrete insulin in a regulated manner are a major focus of current diabetes research. In particular, recent discoveries of the detailed role and requirements for reorganization/remodeling of filamentous actin (F-actin) in the regulation of insulin release from the β cell have appeared at the forefront of islet function research, having lapsed in prior years due to technical limitations. Recent advances in live-cell imaging and specialized reagents have revealed localized F-actin remodeling to be a requisite for the normal biphasic pattern of nutrient-stimulated insulin secretion. This review will provide an historical look at the emergent focus on the role of the actin cytoskeleton and its regulation of insulin secretion, leading up to the cutting-edge research in progress in the field today.
Related collections
Most cited references160
- Record: found
- Abstract: found
- Article: not found
SNAREs--engines for membrane fusion.
- Record: found
- Abstract: found
- Article: not found
A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion.
- Record: found
- Abstract: found
- Article: not found