- Record: found
- Abstract: found
- Article: found
Hemorrhagic Transformation After Ischemic Stroke: Mechanisms and Management
Read this article at
Abstract
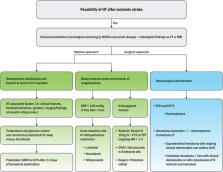
Symptomatic hemorrhagic transformation (HT) is one of the complications most likely to lead to death in patients with acute ischemic stroke. HT after acute ischemic stroke is diagnosed when certain areas of cerebral infarction appear as cerebral hemorrhage on radiological images. Its mechanisms are usually explained by disruption of the blood-brain barrier and reperfusion injury that causes leakage of peripheral blood cells. In ischemic infarction, HT may be a natural progression of acute ischemic stroke and can be facilitated or enhanced by reperfusion therapy. Therefore, to balance risks and benefits, HT occurrence in acute stroke settings is an important factor to be considered by physicians to determine whether recanalization therapy should be performed. This review aims to illustrate the pathophysiological mechanisms of HT, outline most HT-related factors after reperfusion therapy, and describe prevention strategies for the occurrence and enlargement of HT, such as blood pressure control. Finally, we propose a promising therapeutic approach based on biological research studies that would help clinicians treat such catastrophic complications.
Related collections
Most cited references131
- Record: found
- Abstract: found
- Article: not found
Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association
- Record: found
- Abstract: found
- Article: not found