- Record: found
- Abstract: found
- Article: found
Role of Na + and Ca 2+ currents in computational model of in-vitro sigh generation
abstract
4 December 2015
24th Annual Computational Neuroscience Meeting: CNS*2015
18-23 July 2015
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Eupneic breathing in mammals is periodically interrupted by spontaneous augmented
breaths (sighs) that are characterized by a biphasic larger-amplitude inspiratory
burst followed by post-sigh apnea. Previous in vitro studies in newborn rodents have
demonstrated that the respiratory oscillator of the pre-Bötzinger complex (preBötC)
can generate the distinct inspiratory-related motor patterns for both eupnea- and
sigh-like activity [1,2]. However it remains debated whether these two types of inspiratory
activities are produced by the same neuronal population or by distinct sub-networks.
Based on recent in vitro data obtained in the mouse embryo [3], we have built a computational
model consisting of two compartments, one dedicated to sigh generation and the other
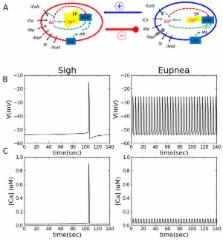
generating eupneic bursts, interconnected through appropriate synapses (Figure 1 A).
Figure 1
Sigh and eupnea activity pattern in silico
. (A) Diagrams of sigh (left) and eupnea (right) network models. Except for the ER
capacity, INaP
and Ih
, the sigh and eupnea models are identical. (B) Intracellular Ca2+
(top) and voltage (bottom) obtained for individual uncoupled compartments (gsyn
=0).
The model reproduces basic features of simultaneous sigh and eupnea generation: two
types of bursts differing in terms of shape, amplitude and frequency of occurrence
and mimics the effect of glycinergic synapses blockade. We designed a two-compartment
computational model for sigh and eupnea subpopulations of neurons with several different
parameters reflecting distinct burst generating mechanisms. The sigh subpopulation
generates a low frequency rhythm based on slow intracellular Ca2+ oscillations and
the eupnea subnetwork generates fast oscillations mainly driven by activation/inactivation
of the persistent Na+ current (Fig 1 B,C).Furthermore, we used this model to make
predictions that were subsequently tested on the isolated preBötC in brainstem slice
preparations. Through a combination of our in vitro and in silico approaches we found
that 1), sigh events are less sensitive to network excitability than eupneic activity,
2)The combination of voltage-gated calcium current and persistent sodium current control
the sigh period of, and 3), specific parameters of Ih activation set the low sensitivity
to excitability in the sigh neuronal subset. Altogether, our results strongly support
the hypothesis that distinct subpopulations within the preBötC network are responsible
for sigh and eupnea rhythmogenesis.
Related collections
Most cited references2
- Record: found
- Abstract: found
- Article: not found
Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength.
S Lieske, Jan-Marino Ramirez (2006)
- Record: found
- Abstract: found
- Article: not found
Emergence of sigh rhythmogenesis in the embryonic mouse.
Coralie Chapuis, Sandra Autran, Gilles Fortin … (2014)