- Record: found
- Abstract: found
- Article: not found
Submicroscopic Gametocytes and the Transmission of Antifolate-Resistant Plasmodium falciparum in Western Kenya
Read this article at
Abstract
Background
Single nucleotide polymorphisms (SNPs) in the dhfr and dhps genes are associated with sulphadoxine-pyrimethamine (SP) treatment failure and gametocyte carriage. This may result in enhanced transmission of mutant malaria parasites, as previously shown for chloroquine resistant parasites. In the present study, we determine the association between parasite mutations, submicroscopic P. falciparum gametocytemia and malaria transmission to mosquitoes.
Methodology/Principal Findings
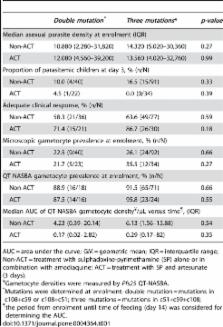
Samples from children treated with SP alone or in combination with artesunate (AS) or amodiaquine were genotyped for SNPs in the dhfr and dhps genes. Gametocytemia was determined by microscopy and Pfs25 RNA–based quantitative nucleic acid sequence–based amplification ( Pfs25 QT-NASBA). Transmission was determined by membrane-feeding assays. We observed no wild type infections, 66.5% (127/191) of the infections expressed mutations at all three dhfr codons prior to treatment. The presence of all three mutations was not related to higher Pfs25 QT-NASBA gametocyte prevalence or density during follow-up, compared to double mutant infections. The proportion of infected mosquitoes or oocyst burden was also not related to the number of mutations. Addition of AS to SP reduced gametocytemia and malaria transmission during follow-up.
Conclusions/Significance
In our study population where all infections had at least a double mutation in the dhfr gene, additional mutations were not related to increased submicroscopic gametocytemia or enhanced malaria transmission. The absence of wild-type infections is likely to have reduced our power to detect differences. Our data further support the use of ACT to reduce the transmission of drug-resistant malaria parasites.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi.
- Record: found
- Abstract: found
- Article: not found
Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria.
- Record: found
- Abstract: found
- Article: not found
